Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
![**Title: Understanding Chemical Reactions and Electrophiles**
**Equation Explanation:**
The provided equation is:
\[ \text{CH}_2 = \text{CH}_2 + \text{Br}^+ \rightarrow +\text{CH}_2-\text{CH}_2\text{Br} \]
This equation represents a step in a chemical reaction where a double-bonded carbon molecule (ethylene, \(\text{CH}_2=\text{CH}_2\)) is reacting with a bromine cation (\(\text{Br}^+\)) to form a bromoalkane product (\(+\text{CH}_2-\text{CH}_2\text{Br}\)).
**Educational Content:**
This is a step in a ___ reaction where ___ is the electrophile.
**Choices for Completion:**
- Radical Br\(^+\)
- Polar Br\(^+\)
- Radical CH\(_2=\)CH\(_2\)
- Polar CH\(_2=\)CH\(_2\)
**Analysis:**
The type of reaction and the identity of the electrophile are to be completed by choosing the correct terms from the options provided.
- **Radical** suggests a reaction involving free radicals, which are atoms or molecules with unpaired electrons.
- **Polar** suggests a reaction involving polar molecules, where there is an uneven distribution of electron density.
**Electrophile Identification:**
The electrophile is the species that accepts an electron pair. In this reaction, \( \text{Br}^+ \) acts as the electrophile. Thus, the blank describing the electrophile should be filled with "Polar Br\(^+\)".
Understanding the nature of reactions and the role of electrophiles is crucial in organic chemistry, especially in mechanisms such as electrophilic addition.](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F14b6b106-4e8a-4775-9d66-77daef426bbe%2Fb16a6575-0c0c-4f58-8585-2324a9398e32%2Fh59emixg_processed.png&w=3840&q=75)
Transcribed Image Text:**Title: Understanding Chemical Reactions and Electrophiles**
**Equation Explanation:**
The provided equation is:
\[ \text{CH}_2 = \text{CH}_2 + \text{Br}^+ \rightarrow +\text{CH}_2-\text{CH}_2\text{Br} \]
This equation represents a step in a chemical reaction where a double-bonded carbon molecule (ethylene, \(\text{CH}_2=\text{CH}_2\)) is reacting with a bromine cation (\(\text{Br}^+\)) to form a bromoalkane product (\(+\text{CH}_2-\text{CH}_2\text{Br}\)).
**Educational Content:**
This is a step in a ___ reaction where ___ is the electrophile.
**Choices for Completion:**
- Radical Br\(^+\)
- Polar Br\(^+\)
- Radical CH\(_2=\)CH\(_2\)
- Polar CH\(_2=\)CH\(_2\)
**Analysis:**
The type of reaction and the identity of the electrophile are to be completed by choosing the correct terms from the options provided.
- **Radical** suggests a reaction involving free radicals, which are atoms or molecules with unpaired electrons.
- **Polar** suggests a reaction involving polar molecules, where there is an uneven distribution of electron density.
**Electrophile Identification:**
The electrophile is the species that accepts an electron pair. In this reaction, \( \text{Br}^+ \) acts as the electrophile. Thus, the blank describing the electrophile should be filled with "Polar Br\(^+\)".
Understanding the nature of reactions and the role of electrophiles is crucial in organic chemistry, especially in mechanisms such as electrophilic addition.
Expert Solution
Step 1
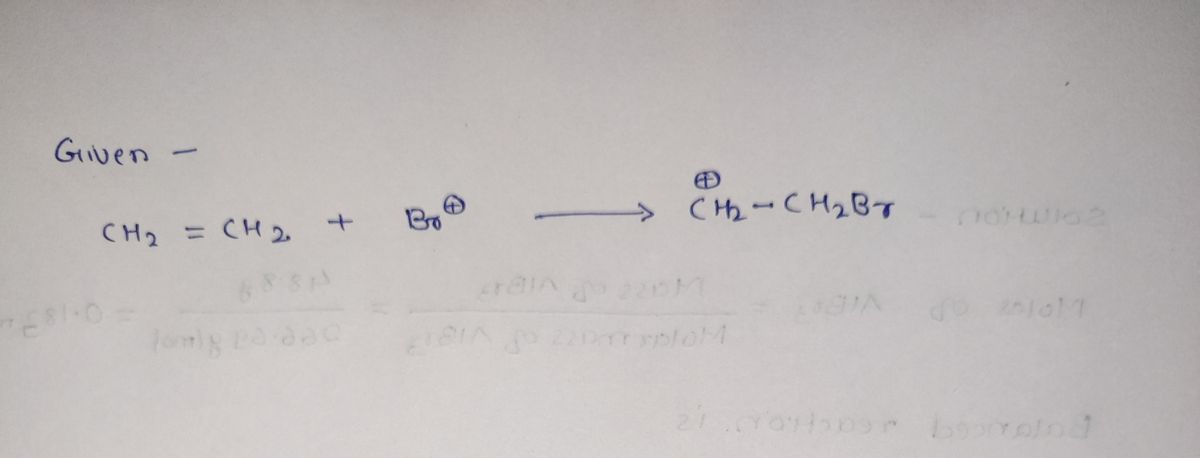
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY