Q: CO(g) + H20(g) 2 C02(g) + H2(g) has an equilibrium constant K, of 0.58 at 1000 °C. If a 80.0-L…
A: Given data,Kc=0.58Initial concentration of CO=0.07mol/LInitial concentration of H2O=0.07mol/LVolume…
Q: At a certain temperature, the equilibrium constant K for the following reaction is 8.1 × 1010 :…
A: Given ,Reaction :
Q: 2 SO2(g) + O2(g) ---> 2 SO3(g). You place 1.00 mol/L each of SO2 and O2 in a flask and…
A: Given :- 2 SO2(g) + O2(g) ---> 2 SO3(g) initial concentration of SO2 = 1.00 mol/L or 1.00…
Q: The following acid-base reaction occurs spontaneously in the gas phase: NH,(g) + HCI(g) = NH,C(s)…
A:
Q: Suppose a 250. mL flask is filled with 1.8 mol of Cl₂, 0.20 mol of CHCl3 and 0.60 mol of HCl. The…
A: First, we need to calculate the initial concentrations of the reactants and products. The…
Q: At a certain temperature, the equilibrium constant K for the following reaction is 7.9 × 10 H, (g) +…
A: as the chemical reaction does not goes 100 completion so at some point the rate of forward reaction…
Q: Suppose a 500. mL flask is filled with 0.60 mol of H₂ and 2.0 mol of HC1. The following reaction…
A:
Q: The equilibrium constant, K, for the following reaction is 9.52×102 at 350 K: CH4(g) +…
A: [CH4]= 0.179M[CCl4] =0.179M[CH2Cl2]= 0.0553MExplanation:
Q: Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid…
A: Information about the question
Q: A chemist is studying the following equilibirum, which has the given equilibrium constant at a…
A: Answer:For any given reaction, value of equilibrium constant KP is equal to the ratio of pressure of…
Q: A chemist is studying the following equilibirum, which has the given equilibrium constant at a…
A: The equilibrium reaction taking place is given as, Given : Initial pressure of N2 = 9.0 atm And…
Q: The equilibrium constant, Ke, for the following reaction is 9.52x102 at 350 K. CH4 (g) + CCI4 (g)=2…
A: Given, the equilibrium chemical reaction as follows,CH4(g)+CCl4(g)⇌2CH2Cl2(g)equilibrium constant…
Q: Suppose a 500. ml. flask is filled with 1.3 mol of H₂O, 0.30 mol of CO₂ and 1.8 mol of H₂. The…
A: The given equilibrium reaction is ' CO(g) + H2O(g) CO2(g) +…
Q: Suppose a 250.mL flask is filled with 0.50mol of Cl2, 0.70mol of HCl and 2.0mol of CCl4. The…
A: A reaction is said to at equilibrium if the forward reaction rate is equal to the rate of backward…
Q: Suppose a 250. mL flask is filled with 1.6 mol of Cl,, 0.60 mol of HCl and 1.0 mol of CCl. The…
A:
Q: information to complete the following table. Suppose a 47. L reaction vessel is filled with 0.56 mol…
A:
Q: At a certain temperature, the equilibrium constant K for the following reaction is 8.92 × 10: NO3(g)…
A:
Q: Suppose a 500. mL flask is filled with 1.7 mol of Cl₂, 0.50 mol of CHCl3 and 1.5 mol of HCl. The…
A: Here we are required to find the equilibrium molarity of CHCl3 when the equilibrium constant value…
Q: At a certain temperature, the equilibrium constant K for the following reaction is 6.35 × 10-8:…
A: The explanation is given below-
Q: Phosphorus pentachloride decomposes according to the chemical equation PC1, (g) PC13(g) + Cl₂(g) Kc…
A: Given :Initial moles of PCl5 = 0.3200 molesVolume the reaction vessel =3.80 LChemical reaction: We…
Q: Write the equilibrium constant expressions, K, and predict the position of equilibrium for the…
A:
Q: The compound NOCl decomposes to nitric oxide and chlorine according to the following equation: 2…
A: Given, 2 NOCl (g) → 2 NO (g) + Cl2 (g) Initially moles of NOCl = 0.510 mol Volume of the flask =…
Q: The equilibrium constant K, for the equation 2H2(g) + CO(g)=CH;OH(g) is 35 at a certain temperature.…
A:
Q: Suppose a 500. mL flask is filled with 0.20 mol of Br,, 1.1 mol of OCl, and 1.8 mol of BrCl. The…
A:
Q: Carbon monoxide and hydrogen react according to the following equation: CO(g) + 3H, (g) - CH4 (9) +…
A: Assuming y moles of CO reacted as per the above reaction. Since from the above reaction we can see…
Q: :a certain temperature, the equilibrium constant K for the following reaction is 2.07 × 10´: H,(g) +…
A: Description: Equilibrium reaction is given:
Q: Nitrogen and oxygen react to produce nitric oxide according to the following equation: N2 (g) + O2…
A:
Q: N2(g) + 202(g) → 2NO2(g) A chemist added 0.35 mol of nitrogen gas to 0.45 mol of oxygen gas in 1.0 L…
A: It is given that the initial moles of N2 are 0.35 moles and of O2 are 0.45 moles and the volume of…
Q: At a particular temperature 8.0 mol NO, gas is placed in a 1.0-L container. Over time the NO2…
A: 2 NO2 (g) ⇔ 2 NO(g) + O2 (g) Initial 8 mol…
Q: Consider the decomposition reaction: X(g) + 2 Y(g) XY2(s) where X and Y are some chemical groups. At…
A: Answer: Value of equilibrium constant is equal to the ratio of molar concentration of products and…
Q: At a certain temperature, the equilibrium constant, ?c, for this reaction is 53.3.…
A:
Q: For the equilibrium: 2 BrCl (g) --> Br2 (g) + Cl2 (g) at 205 °C, the equilibrium constant, Kc, is…
A:
Q: At a certain temperature, the equilibrium constant K for the following reaction is 1.1: Cl₂(g) +…
A:
Q: At a certain temperature, the equilibrium constant K for the following reaction is 3.44 x 10°:…
A: The equilibrium reaction given is,
Q: A mixture of 0.10 mole NO, 0.050 mole H₂, and 0.10 mole H₂O is placed in a 2.0 L vessel. The…
A: Given -> Initial mole of NO = 0.10 mole Initial mole of H2 = 0.050 mole Initial mole of H2O =…
Q: For the reaction: 2CO2 (g) = 2CO(g) + O2(g), What can be said about this reaction at this…
A:
Q: At a certain temperature, the equilibrium constant K for the following reaction is 6.8 × 10°: Cl,(2)…
A: The reaction is as follows: Cl2(g) + CHCl3(g) ⇌HCl(g) + CCl4(g)........................(1) The…
Q: At a certain temperature, the equilibrium constant K for the following reaction is 5.96 × 10¹: H₂(g)…
A: The balanced chemical equation is as follows: ...(1)The value of the equilibrium constant is The…
Q: Consider the following equilibrium system at 355 K. 2NOBr(g) -----> 2NO(g)+Br2(g). If an equilibrium…
A: [NOBr] = 3.21 x 10-2 M [NO] = 2.01 x 10-2 M [Br2] = 3.98 x 10-2 M Keq = ?
Q: Suppose a 500. mL flask is filled with 0.30 mol of Br₂, 1.2 mol of OCI, and 0.60 mol of BrCl. The…
A: The objective is the computation of equilibrium molarity of Br2, i.e., [Br2]. Equilibrium molarity…
The following reaction is important in the manufacture of sulfuric acid:
SO2(g)+1/2 O2(g)->SO3(g)
At a certain temperature, 3.61 x10^-2 mol of SO2 and 3.68X10^-2 mol of O2 are sealed in a 1.00-L reaction vessel. When equilibrium is reached, the concentration of SO3 is determined to be 2.98X10^-2. Calculate Kc for this reaction.
Kc=
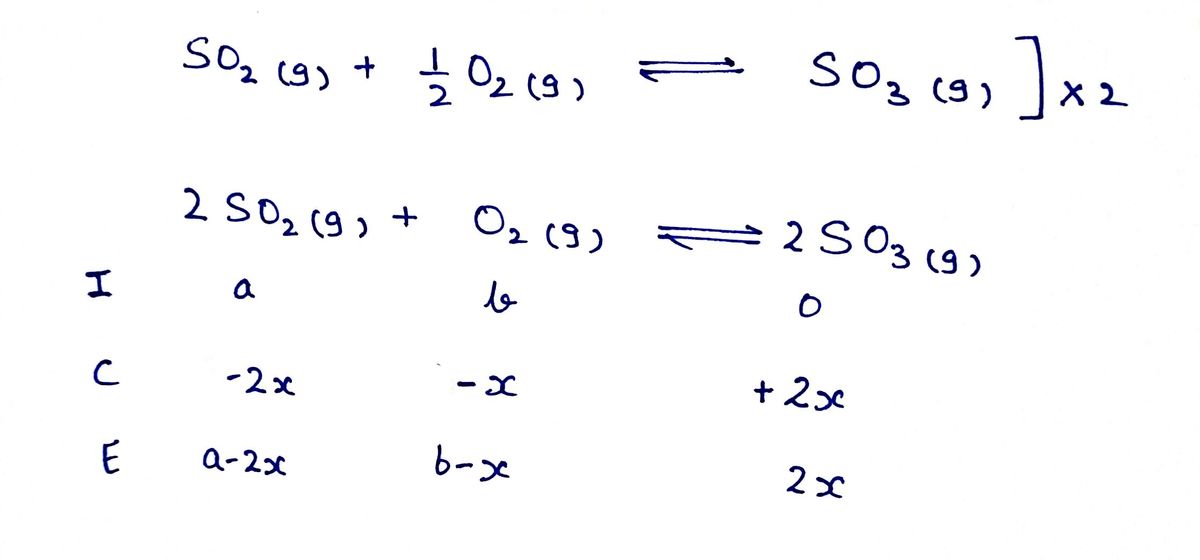
Step by step
Solved in 2 steps with 2 images

- Using the general properties of equilibrium constants 0/5 At a certain temperature, the equilibrium constant K for the following reaction is 7.4 × 10 ': N,(e) + 0,(g) -2NO(g) Use this information to complete the following table. Suppose a 35. L reaction vessel is filled with 1.7 mol of NO. What can you say about the composition of the mixture in the vessel at equilibrium? O There will be very little N, and 0,. O There will be very little NO. O Neither of the above is true. What is the equilibrium constant for the following reaction? Round your answer to 2 significant digits. K =] 2 NO(g) N2(0)+O2(a) 1, What is the equilibrium constant for the following reaction? Round your answer to 2 significant digits. K = ] 2N,(0)+20,(0) 4 NO(g) 1,At a certain temperature, the equilibrium constant K for the following reaction is 0.71: N2(g) + O2(g) =2 NO(g) Use this information to complete the following table. Suppose a 43. L reaction vessel is filled with 1.0 mol of NO. What can you say about the composition of the mixture in the vessel at equilibrium? There will be very little N2 and 02. There will be very little NO. Neither of the above is true. What is the equilibrium constant for the following reaction? Be sure your answer has the correct number of significant digits. K =0 2 NO(g) N,(9)+O2(9) What is the equilibrium constant for the following reaction? Be sure your answer has the correct number of significant digits. K = 3 N2(9)+30,(9) 6 NO(g)Calculating an equilibrium constant from a partial equilibrium composition Steam reforming of methane (CH4) produces "synthesis gas," a mixture of carbon monoxide gas and hydrogen gas, which is the starting point for many important industrial chemical syntheses. An industrial chemist studying this reaction fills a 75.0 L tank with 35. mol of methane gas and 16. mol of water vapor, and when the mixture has come to equilibrium measures the amount of hydrogen gas to be 43. mol. Calculate the concentration equilibrium constant for the steam reforming of methane at the final temperature of the mixture. Round your answer to 2 significant digits. K = [] с x10 X Ś ? olo 18 Ar
- Steam reforming of methane ( CH, ) produces "synthesis gas," a mixture of carbon monoxide gas and hydrogen gas, which is the starting point for many important industrial chemical syntheses. An industrial chemist studying this reaction fills a 5.0 L flask with 0.93 atm of methane gas and 2.7 atm of water vapor, and when the mixture has come to equilibrium measures the partial pressure of carbon monoxide gas to be 0.47 atm. Calculate the pressure equilibrium constant for the steam reforming of methane at the final temperature of the mixture. Round your answer to 2 significant digits. K_ = || x10Suppose a 250. mL flask is filled with 1.2 mol of Br,, 1.4 mol of OCl, and 1.1 mol of BrCl. The following reaction becomes possible: Br, (g) +OCl, (g) - BrOC1 (g) +BrC1(g) The equilibrium constant K for this reaction is 0.483 at the temperature of the flask. Calculate the equilibrium molarity of OCl,. Round your answer to two decimal places. OMSuppose a 250. mL flask is filled with 1.6 mol of Br₂, 0.70 mol of OC12 and 0.50 mol of BrCl. The following reaction becomes possible: Br₂(g) + OC1₂(g) → BrOC1 (g) + BrCl(g) The equilibrium constant K for this reaction is 2.87 at the temperature of the flask. Calculate the equilibrium molarity of Br₂. Round your answer to two decimal places. M Ś
- A gaseous mixture contains 0.27 mol CO, 0.12 mol H2, and 0.022 mol H,O, plus an unknown amount of CH4, in each liter. This mixture is at equilibrium at a certain temperature. CO(9) + 3H, (9) = CH4 (9) + H,O(9) What is the concentration of CH4 in this mixture? The equilibrium constant K, equals 3.99.Using the general properties of equilibrium constants At a certain temperature, the equilibrium constant K for the following reaction is 924.: CO(g) + H₂O(g) → CO₂(g) + H₂(g) Use this information to complete the following table. Suppose a 17. L reaction vessel is filled with 0.75 mol of CO₂ and 0.75 mol of H₂. What can you say about the composition of the mixture in the vessel at equilibrium? What is the equilibrium constant for the following reaction? Round your answer to 3 significant digits. CO₂(g) + H₂(9) CO(g)+H₂O(g) What is the equilibrium constant for the following reaction? Round your answer to 3 significant digits. 3 CO(g) + 3H₂O(g) P 3 CO₂(g) + 3H₂(9) There will be very little CO and H₂O. There will be very little CO2 and H₂. Neither of the above is true. K = 0 K = 0 ■ x10 X ? 00. Ar 8.Suppose a 500. mL flask is filled with 0.20 mol of Br,, 1.6 mol of OCl, and 1.9 mol of BrOCl. The following reaction becomes possible: 2' Br, (g) +OCl, (g)- BROCI(g)+BrC1(g) The equilibrium constantK for this reaction is 5.57 at the temperature of the flask. Calculate the equilibrium molarity of BrOCl. Round your answer to two decimal places. O M
- Phosphorus pentachloride decomposes according to the chemical equation PC15(g) PC13(g) + Cl₂(g) Kc = 1.80 at 250 °C A 0.1584 mol sample of PC1, (g) is injected into an empty 2.00 L reaction vessel held at 250 °C. Calculate the concentrations of PCl, (g) and PC13 (g) at equilibrium.Using the general properties of equilibrium constants At a certain temperature, the equilibrium constant k for the following reaction is 9.84 x 101: N,(g) + 0,(g) = 2 NO(g) Use this information to complete the following table. Suppose a 30. L reaction vessel is filled with 0.64 mol of N2 and There will be very little N, and O2. alo 0.64 mol of 0,. What can you say about the composition of the mixture in the vessel at equilibrium? There will be very little NO. Neither of the above is true. What is the equilibrium constant for the following reaction? Round your answer to 3 significant digits. K 2 NO(g) N3(9)+Og(9) What is the equilibrium constant for the following reaction? Round your answer to 3 significant digits. K = 3 N,(9)+30,(9) 6 NO(g)N2(g) and O2(g) can exist in equilibrium with NO(g), as shown below. The equilibrium constant at 25.0°C is 4.8 x 10-31. If initially there are 1.35 mol of nitrogen and 0.60 mol of oxygen in a 2.00 L vessel, find the equilibrium concentrations of each species. N2(g) + O2(g) → 2NO(g)











