Calcium in a 100.0 mL sample of fuit juice was analyzed by an electrochemical method that gave a detector current of 2.40 mA. A standard addition of 10.00 mL of 80.0mg/mL calcium ion increased the current to 4.56 mA. Find the concentration of calcium in the fruit juice.

Any particular substance/compound's quantity either in moles or in mass existing into solution's defined volume or mass is basically addressed as "concentration". The concentration gets analyzed in various forms like g/L, mol/L, mg/mL and so on.
Given
The volume of sample of fruit juice is 100.0 mL.
The initial current is 2.40 mA.
The increased (final) current is 4.56 mA.
The volume of standard calcium ion is 10.00 mL.
The concentration of standard calcium ion is 80.0 mg/mL.
The equation relating the substance mass with the current before any standard addition of calcium (present in fruit juice) is shown below.
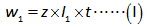
The equation relating the substance mass with the current after the standard addition of calcium is shown below.
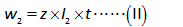
Divide the equation (I) by (II).
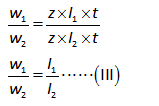
The mass of added calcium ion (w2) can be calculated as shown below.
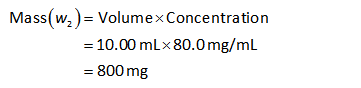
Substitute the known values in the equation (III).
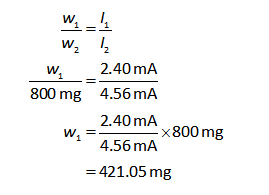
Step by step
Solved in 5 steps with 6 images









