and the amount of lactase enzyme in their gut diminishes. Since the enzyme is commonly found in the gut of humans it can withstand a pH that is very acidic, ranging from a pH of 2-7. A student decided to do an
Lactase is an enzyme that breaks down the sugar lactose which is commonly found in dairy products. Some people develop lactose intolerance as they grow older and the amount of lactase enzyme in their gut diminishes. Since the enzyme is commonly found in the gut of humans it can withstand a pH that is very acidic, ranging from a pH of 2-7. A student decided to do an experiment to determine how the pH of a solution affects lactase enzyme activity. Lactase works by breaking lactose into its two component sugars of glucose and galactose.
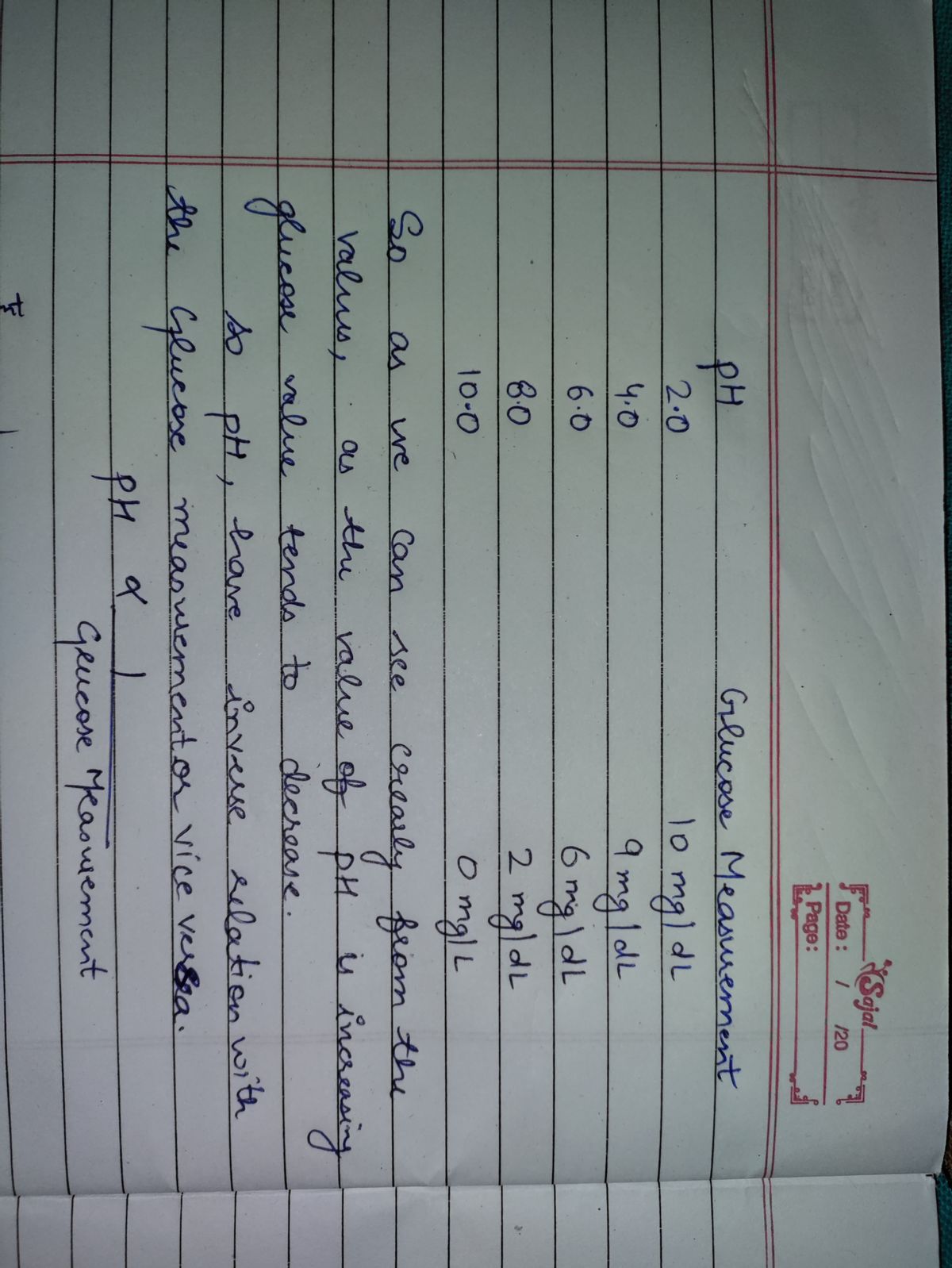
To conduct the experiment the student prepared a solution of lactose and placed it into 5 different test tubes. A pH buffer solution was then added to each test tube with a pH of 2.0 in the first tube, 4.0 in the second, 6.0 in the third, 8.0 in the fourth, and a 10 pH in the fifth. Next, the enzyme lactase was added in equal amounts to each test tube and allowed to sit for ten minutes white the enzyme reacted with the lactose. At the end of the ten minutes, a droplet from each test tube was tested using a glucose meter which measured the amount of glucose present in the solution. This measurement was used to gauge enzyme activity, the higher the glucose the more active the enzyme was. This experiment was repeated 2 more times for a total of 3 trials. An average was then calculated.
The average amount of glucose recorded for each pH was as follows; a pH of 2.0 had a glucose measurement of 10mg/dL, a pH of 4.0 had a glucose measurement of 9mg/dL, the pH of 6.0 had a glucose measurement of 6mg/dL, the pH of 8.0 had a glucose measurement of 2mg/dL and the pH of 10.0 had a glucose measurement of 0mg/dL.
Create a graph that would be used to show how the pH affected the glucose levels.
Step by step
Solved in 2 steps with 2 images









