A solution contains 5.43x10~³ M silver acetate and 1.22×10-² M zinc nitrate. Solid sodium carbonate is added slowly to this mixture. What is the concentration of zinc ion when silver ion begins to precipitate? [Zn?*] ={ M %3D
Q: A loss of an oxide atom results in a 'vacancy'. The presence of these vacancies is required for…
A: Nonstoichiometric compounds have variable stoichiometries over a given range with no dramatic change…
Q: QUESTION 7 A battery is made up of an unknown number of cells. Each of the cells in the battery is…
A:
Q: A student collected 38.62 mL of H2 over water at 25.00 ºC. The water level inside the collection…
A: The reaction of Zn with the HCl is produced ZnCl2 and H2 Zn + HCl ------------> ZnCl2 + H2 from 1…
Q: 1. A laboratory requires 2000 cm° of an antifreeze solution consisting of 30 mol % methanol in…
A: To solve this problem, we can use the concept of partial molar volumes and mole fraction. Let's…
Q: A sample of pure sodium has a mass of 15.2 g. Calculate the number of moles in the sample and sodium…
A: Given, mass of the sodium, m = 15.2 g
Q: Question 2 The following table shows the ICF and ECF volumes, plus the osmolarity at osmotic…
A: Given The following table shows the ICF and ECF volumes, plus the osmolarity at osmotic equilibrium,…
Q: Ag(s) + PbSO(s) → Pb(s) + Ag* (ac) + So²² (ac). Datos AH 298: 53, 22 Kcal :53,41cal 4 AG 298: Cal…
A:
Q: a) What is the average K.E. of molecules in air at latm pressure and 20C? 6) b) What is the average…
A: Average energy of molecules in air is given by, E = (3/2)* KT It depends only on temperature only.…
Q: An irregularly shaped piece of metal of density 7.8g/cm3 weighs 429 grams when completely immersed…
A: Archimedes' principle states that when an object is fully or partially immersed in a fluid, it…
Q: One component of a metal sculpture consists of a solid cube with an edge of length 0.201 m. The…
A: Given:- Edge of cube = 0.201 density of cube = 6110 kg/m3
Q: You must have to submit the correct answer to get a helpful rating.
A: Thank you.
Q: What is the molar mass of a gas sample that has a density 0.906 g/L at 42.0°C and 1.16 atm? Select…
A: Since we have to solve one question per session, solution for the first question is provided here.…
Q: 2) The Mark-Houwink-Sakurada (MHS) equation allows us to relate the intrinsic viscosity, [n], of a…
A: Method to solve the question is in the explanation- Explanation: Logarithm of M vs. Logarithm of [n]…
Q: Write the molecular equation and net ionic equation for the reaction of hydroiodic acid and…
A: Acid-base reaction between strong acid HI and strong base KOH is : Molecular equation is : HI…
Q: An ideal rectifying contact is formed by depositing gold on n-type silicon doped at 1015 cm-3. At T…
A:
Q: After an injection of Drug Y, the concentration of the drug in the bloodstream drops at the contin…
A:
Q: A continuous drip of an antidysrhythmic medication is ordered at a rate of 2 mg/min. The standard…
A: A continuous drip of an antidysrhythmic medication is ordered at a rate of 2 mg/min i.e.120 mg/hr If…
Q: A sample of pure sodium has a mass of 11.0 g. Calculate the number of moles in the sample and sodium…
A:
Q: A particular chemical reaction occurs at room temperature (293 K) at half the rate that it does at…
A:
Q: The force constant of HCl is 0.0482 N/m. a) Calculate its vibrational frequency. b) How much does…
A: Given
Q: What is the mass of liquid sample if the volume is 20.0 mL and has a density 1.28 g/mL? 25.6 g O…
A: Given data : Density is represented by ρ Volume is represented by V Required : Mass
Q: Aqua regia is an acid mixture that can be used to dissolve gold. Aqua regia has a density of 1210.…
A: Given data: The density of Aqua regia is ρA=1210 kg/m3. The density of gold is ρg=19.30 g/cm3. The…
Q: A hypothetical metal has the simple cubic crystal structure. If its atomic weight is 74.5 g/mole and…
A: The coordinate number for any crystal is defined as the number of nearest neighbors in the unit cell…
Q: 6.a)What do covalently bonded atoms form? __________________________ b) Describe the…
A:
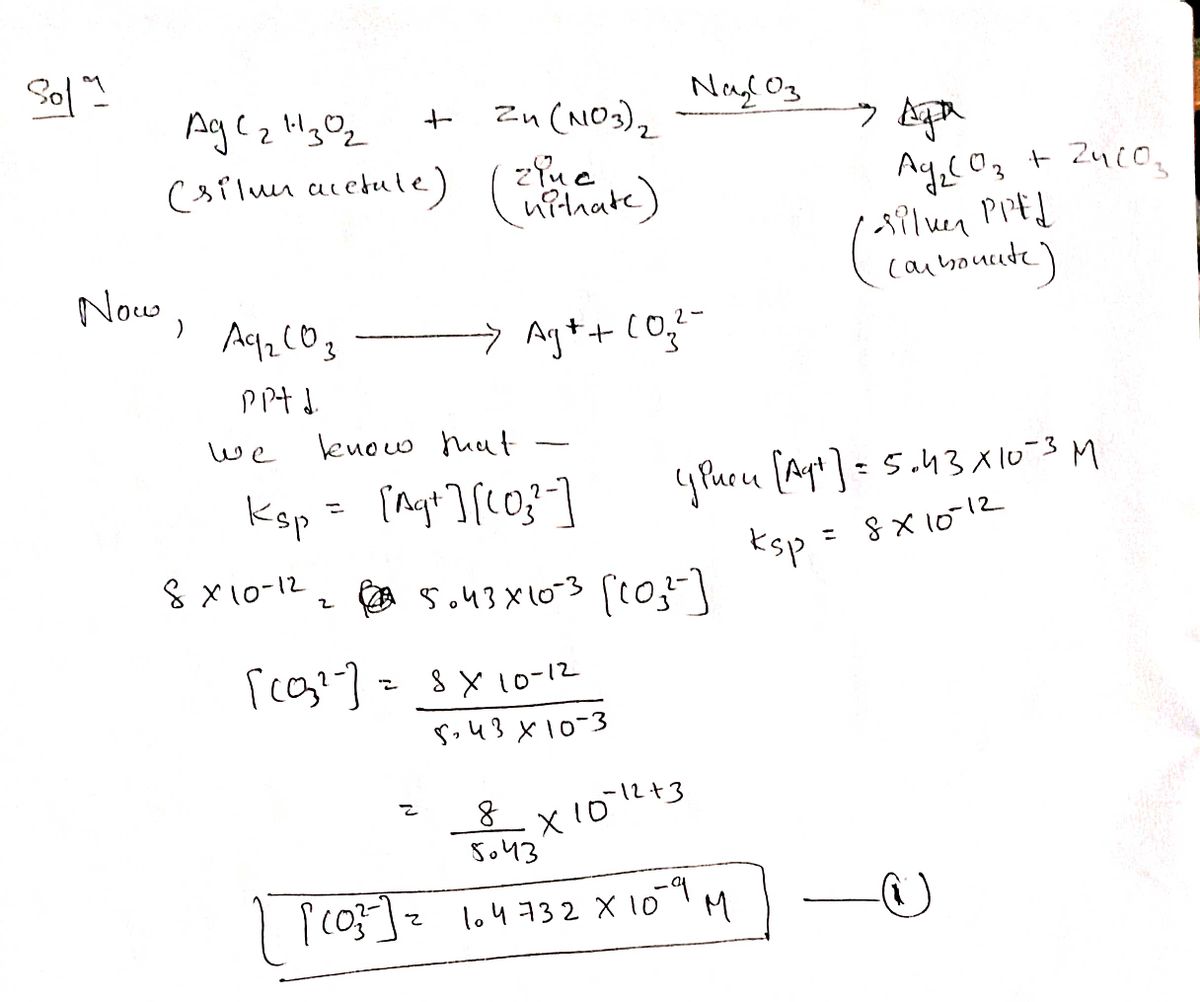
![A solution contains 5.43x103 M silver acetate and 1.22x10-2 M zinc nitrate.
Solid sodium carbonate is added slowly to this mixture.
What is the concentration of zinc ion when silver ion begins to precipitate?
[Zn2*] =(](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Fe4d4a826-3cd8-448d-b0f0-2ac66e032b84%2F0cba593d-b24a-4dac-aa02-79d30b0d42cf%2F4m0oas_processed.jpeg&w=3840&q=75)
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

- 1. Calculate Z and V for ethylene at 25°C and 12 bar by the following equations: The truncated virial equation with the following experimental values of virial coefficients: B = −140 cm 3 ・mol−1, C = 7200 cm6 ・mol−2 ?=1 + B/V + C/V2 The truncated virial equation, Z = 1 + BP/RT with a value of B from the generalized Pitzer correlation for the second virial coefficient. The Redlich/Kwong equation The Soave/Redlich/Kwong equation The Peng/Robinson equationA metal ore consist of element X and Y with relative aboundance in volume of 20% and 80% respectively. If the densities of X and Y are respectively 6.5 and 4.5 what is the densities of the metal ore?A sample of pure sodium has a mass of 17.4 g. Calculate the number of moles in the sample and sodium atoms in the sample. HINT (a) moles in the sample moles (b) sodium atoms in the sample atoms
- Two large tanks, each holding 100L of liquid, are interconnected by pipes, with the liquid flowing from tank A into tank B at a rate of 3L/min and from B into A at a rate of 1L/min. The liquid inside each tank is kept well stirred. A brine solution with a concentration of 0.1kg/L of salt flows into tank A at a rate of 6L/min. The (diluted) solution flows out of the system from tank A at 4L/min and from tank B at 2L/min. If initially, tank A contains pure water and tank B contains 10kg of salt, determine the mass of salt in each tank at time t≥0. What is the solution to the system? x(t)= y(t)=helpA composite material contains 15% TiC, 10% Co, 20% TaC and 55% WC. The densities of the components are given as Pric = 4.9 gcm³, pco = 8.9 gcm³, PTac = 14.5 gcm³, Pwc = 15.8 gcm³³. Estimate the density of the composite.
- A sample of pure sodium has a mass of 15.7 g. Calculate the number of moles in the sample and sodium atoms in the sample. HINT (a) moles in the sample moles (b) sodium atoms in the sample atomsOne component of a metal sculpture consists of a solid cube with an edge of length 36.9 cm. The alloy used to make the cube has a density of 6610 kg/m³. Find the cube's mass. m = kgHelp me please