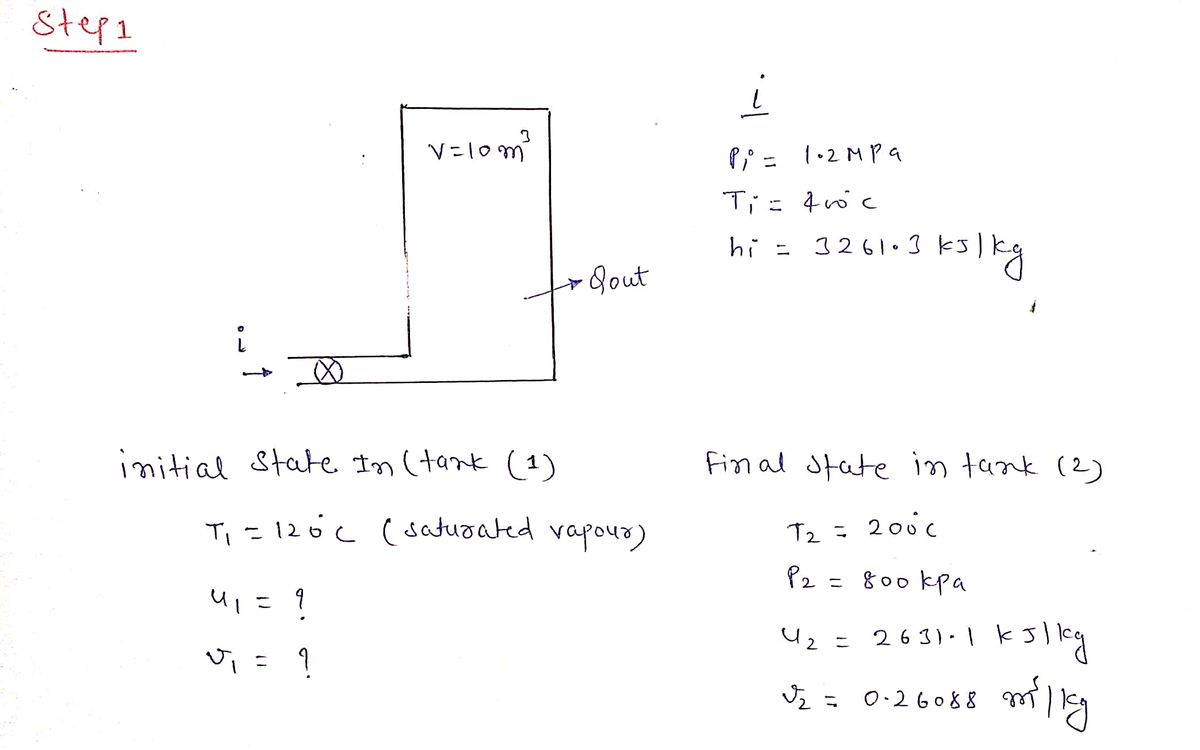
A rigid tank of volume 10 m³ initially contains saturated water vapor at a temperature of 120 °C. Steam at a pressure 1.2 MPa and a temperature of 400 °C enters the tank through a valve in steam line that is connected to the tank until the final pressure in the tank is 800 kPa, at which time the temperature is 200 °C. All kinetic and potential energy effects can be neglected. A schematic of the problem and properties at all state points except state 1 are shown in the figure below. All of the properties at state 2 and the inlet state i are provided on the figure. Initial State in Tank T₁-120 °C, Sat. vapor u₁=? kJ/kg V₁=? m³/kg Pi=1.2 MPa, Ti-400 °C hi-3261.3 kJ/kg V=10 m³ Final State in Tank T: 200 °C, P₂-800 kPa u₂=2631.1 kJ/kg v₂=0.26088 m³/kg Qout
A rigid tank of volume 10 m³ initially contains saturated water vapor at a temperature of 120 °C. Steam at a pressure 1.2 MPa and a temperature of 400 °C enters the tank through a valve in steam line that is connected to the tank until the final pressure in the tank is 800 kPa, at which time the temperature is 200 °C. All kinetic and potential energy effects can be neglected. A schematic of the problem and properties at all state points except state 1 are shown in the figure below. All of the properties at state 2 and the inlet state i are provided on the figure. Initial State in Tank T₁-120 °C, Sat. vapor u₁=? kJ/kg V₁=? m³/kg Pi=1.2 MPa, Ti-400 °C hi-3261.3 kJ/kg V=10 m³ Final State in Tank T: 200 °C, P₂-800 kPa u₂=2631.1 kJ/kg v₂=0.26088 m³/kg Qout
Elements Of Electromagnetics
7th Edition
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Sadiku, Matthew N. O.
ChapterMA: Math Assessment
Section: Chapter Questions
Problem 1.1MA
Related questions
Question
![## Mass and Energy Balance Questions
### Mass Balance
**Question:**
By making a mass balance on the system, the mass of the steam that entered the tank, \( m_i \), in kg is
\[ \_\_\_\_\_\_\_\_\_ \]
### Energy Balance
**Question 11:**
By making an energy balance on the system, the heat transferred during the process in kJ is
\[ \_\_\_\_\_\_\_\_\_ \]
### Heat Transfer
**Question 12:**
The heat transfer is:
- \( \circ \) Partly into and partly out of the system.
- \( \circ \) 0
- \( \circ \) Into the system
- \( \circ \) Out of the system
Use the options provided to evaluate the direction and magnitude of heat transfer in the system based on the analysis of energy exchange.](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F0caa681c-a86e-42ac-a3af-b6c8d3fe3f57%2F0fbbd860-f177-41bc-a9c5-3b6a28e67072%2F73psy06_processed.jpeg&w=3840&q=75)
Transcribed Image Text:## Mass and Energy Balance Questions
### Mass Balance
**Question:**
By making a mass balance on the system, the mass of the steam that entered the tank, \( m_i \), in kg is
\[ \_\_\_\_\_\_\_\_\_ \]
### Energy Balance
**Question 11:**
By making an energy balance on the system, the heat transferred during the process in kJ is
\[ \_\_\_\_\_\_\_\_\_ \]
### Heat Transfer
**Question 12:**
The heat transfer is:
- \( \circ \) Partly into and partly out of the system.
- \( \circ \) 0
- \( \circ \) Into the system
- \( \circ \) Out of the system
Use the options provided to evaluate the direction and magnitude of heat transfer in the system based on the analysis of energy exchange.

Transcribed Image Text:### Problem Statement
A rigid tank with a volume of 10 m³ initially contains saturated water vapor at a temperature of 120 °C. Steam, at a pressure of 1.2 MPa and a temperature of 400 °C, enters the tank through a valve. The tank's connection allows steam entry until the final pressure inside the tank reaches 800 kPa. At this point, the temperature is 200 °C.
Neglect all kinetic and potential energy effects. A schematic of the problem with properties at all state points is provided, except for state 1. The properties at state 2 and the inlet state "i" are shown below.
### Schematic Diagram and Properties
#### Initial State in Tank
- **Temperature (T₁):** 120 °C (Saturated vapor)
- **Specific internal energy (u₁):** Unknown (kJ/kg)
- **Specific volume (v₁):** Unknown (m³/kg)
#### Inlet State
- **Pressure (Pᵢ):** 1.2 MPa
- **Temperature (Tᵢ):** 400 °C
- **Specific enthalpy (hᵢ):** 3261.3 kJ/kg
#### Final State in Tank
- **Temperature (T₂):** 200 °C
- **Pressure (P₂):** 800 kPa
- **Specific internal energy (u₂):** 2631.1 kJ/kg
- **Specific volume (v₂):** 0.26088 m³/kg
A diagram illustrates the tank with the specified volume, alongside the condition of heat transfer out of the system, represented by "Q_out."
This text and diagram provide essential conditions and parameters needed to solve the problem regarding the thermodynamic processes occurring within the system.
Expert Solution
Step 1
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Elements Of Electromagnetics
Mechanical Engineering
ISBN:
9780190698614
Author:
Sadiku, Matthew N. O.
Publisher:
Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:
9780134319650
Author:
Russell C. Hibbeler
Publisher:
PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:
9781259822674
Author:
Yunus A. Cengel Dr., Michael A. Boles
Publisher:
McGraw-Hill Education

Elements Of Electromagnetics
Mechanical Engineering
ISBN:
9780190698614
Author:
Sadiku, Matthew N. O.
Publisher:
Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:
9780134319650
Author:
Russell C. Hibbeler
Publisher:
PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:
9781259822674
Author:
Yunus A. Cengel Dr., Michael A. Boles
Publisher:
McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:
9781118170519
Author:
Norman S. Nise
Publisher:
WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:
9781337093347
Author:
Barry J. Goodno, James M. Gere
Publisher:
Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:
9781118807330
Author:
James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:
WILEY