A process to purify glycerine from 10% to 25% is composed of an extraction tower and a distillation column. The inlet to the extraction tower has a flowrate of 1000 lb/hr and contains 10 wt% glycerine, 3% NaCl and the balance is water. Alcohol solution is also fed to the extraction tower at a flowrate of 1000 lb/hr and is 98% butyl alcohol in water. The discarded bottom outlet of the extraction column contains all of the input NaCl, as well as 1% each of glycerine and butyl alcohol. The top outlet from the extraction tower is fed to a distillation column. The top product from the distillation column contains only butyl alcohol and 5% water. The bottom product stream contains 25% glycerine and the balance water. Calculate the glycerine output flowrate (lb/hr) of the bottom product of the distillation column.
A process to purify glycerine from 10% to 25% is composed of an extraction tower and a distillation column. The inlet to the extraction tower has a flowrate of 1000 lb/hr and contains 10 wt% glycerine, 3% NaCl and the balance is water. Alcohol solution is also fed to the extraction tower at a flowrate of 1000 lb/hr and is 98% butyl alcohol in water. The discarded bottom outlet of the extraction column contains all of the input NaCl, as well as 1% each of glycerine and butyl alcohol. The top outlet from the extraction tower is fed to a distillation column. The top product from the distillation column contains only butyl alcohol and 5% water. The bottom product stream contains 25% glycerine and the balance water. Calculate the glycerine output flowrate (lb/hr) of the bottom product of the distillation column.
The flow sheet of the process can be shown as below
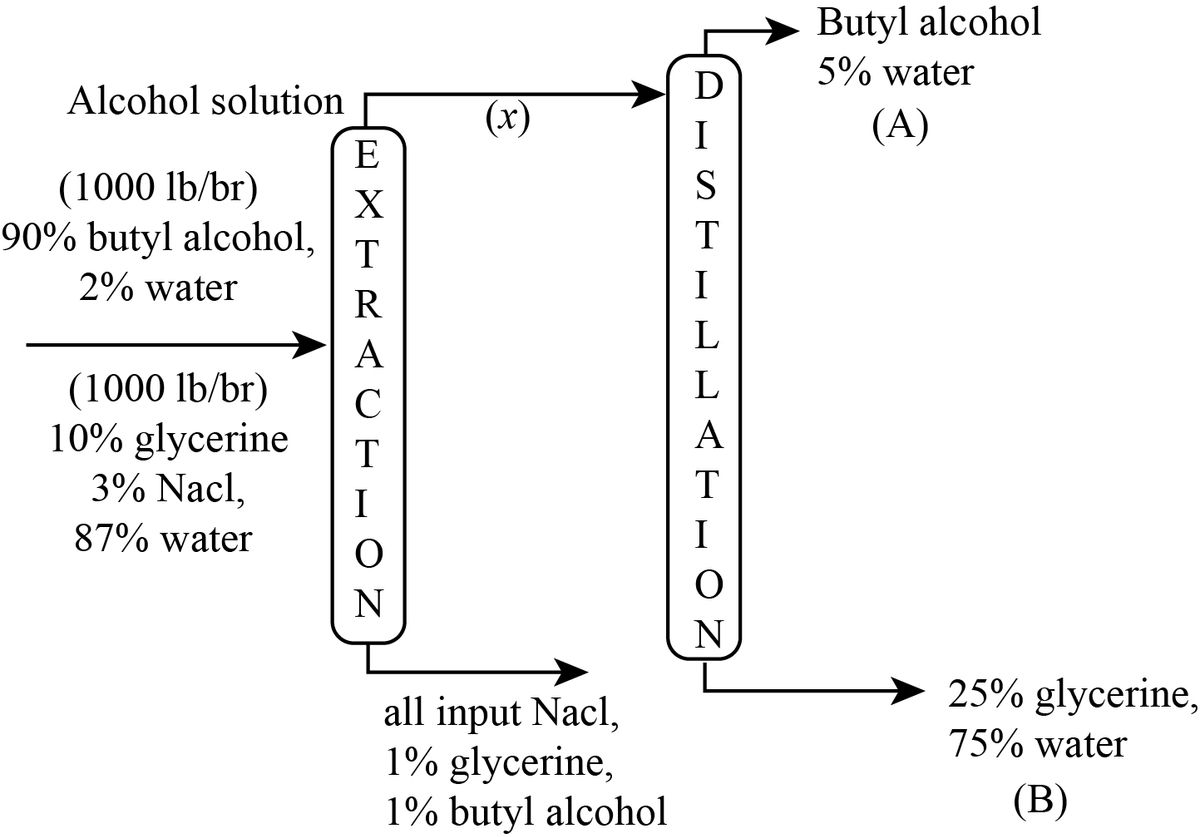
Let x, y be the flow rate of top and bottom stream of extraction column respectively in lb/hr.
Let A, B be the flow rate of the top and bottom stream of distillation column respectively in lb/hr.
In the bottom stream of extraction column, the composition is 98% NaCl, 1% glycerine and 1% butyl alcohol
Applying NaCl balance over extraction column, we get,
Step by step
Solved in 3 steps with 1 images









