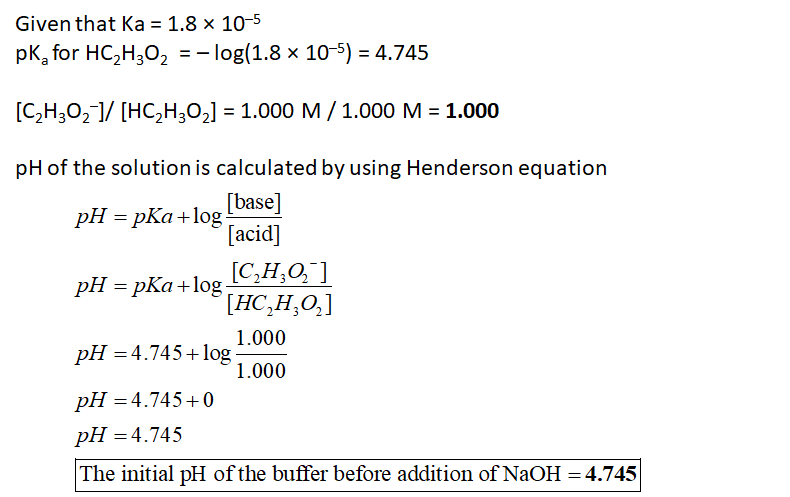
A hypothetical Buffer C contains 1.000 M HC2H3O2 and 1.000 M C,H3O2". Answer the following questions in the text boxes provided with the correct amount of significant figures. K, = 1.8 × 10-5 IMPORTANT: When entering your answers: • Enter the number only (no units) • Do not leave any spaces • Use a leading zero before the decimal when necessary • Report your numbers to the proper significant figures [C>H3O2] / [HC2H,O2] = pHinitial of the buffer = A 20.00 mL sample of Buffer C is combined with 5.00 mL of 0,5000 NAOH. Initial moles of each conjugate (both are the same) mol C2H3O2¯ or HC2H3O2 %3D Moles converted by the NAOH = mol C2H3O2 or HC2H3O2 Total volume = pHinal of the buffer = ApH of the buffer =
A hypothetical Buffer C contains 1.000 M HC2H3O2 and 1.000 M C,H3O2". Answer the following questions in the text boxes provided with the correct amount of significant figures. K, = 1.8 × 10-5 IMPORTANT: When entering your answers: • Enter the number only (no units) • Do not leave any spaces • Use a leading zero before the decimal when necessary • Report your numbers to the proper significant figures [C>H3O2] / [HC2H,O2] = pHinitial of the buffer = A 20.00 mL sample of Buffer C is combined with 5.00 mL of 0,5000 NAOH. Initial moles of each conjugate (both are the same) mol C2H3O2¯ or HC2H3O2 %3D Moles converted by the NAOH = mol C2H3O2 or HC2H3O2 Total volume = pHinal of the buffer = ApH of the buffer =
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
All boxes please
![# Buffer Calculation Exercise
## Problem Statement
A hypothetical Buffer C contains 1.000 M \( \text{HC}_2\text{H}_3\text{O}_2 \) and 1.000 M \( \text{C}_2\text{H}_3\text{O}_2^- \). Answer the following questions in the text boxes provided with the correct amount of significant figures. \( K_a = 1.8 \times 10^{-5} \).
### Important Instructions
- Enter the number only (no units).
- Do not leave any spaces.
- Use a leading zero before the decimal when necessary.
- Report your numbers to the proper significant figures.
## Questions and Calculations
1. **Ratio Calculation**
\[
\frac{[\text{C}_2\text{H}_3\text{O}_2^-]}{[\text{HC}_2\text{H}_3\text{O}_2]} = \_\_\_\_\_\_\_\_
\]
2. **Initial pH Calculation**
\[
\text{pH}_{\text{initial of the buffer}} = \_\_\_\_\_\_\_\_
\]
### Buffer Titration
A 20.00 mL sample of Buffer C is combined with 5.00 mL of 0.5000 M NaOH.
3. **Initial Moles Calculation**
\[
\text{Initial moles of each conjugate (both are the same)} = \_\_\_\_\_\_\_\_ \text{ mol } \text{C}_2\text{H}_3\text{O}_2^- \text{ or } \text{HC}_2\text{H}_3\text{O}_2
\]
4. **Moles Converted Calculation**
\[
\text{Moles converted by the NaOH} = \_\_\_\_\_\_\_\_ \text{ mol } \text{C}_2\text{H}_3\text{O}_2^- \text{ or } \text{HC}_2\text{H}_3\text{O}_2
\]
5. **Total Volume Calculation**
\[
\text{Total volume} = \_\_\_\_\_\_\_\_ \text{ L}
\]](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Fb7468297-7302-4b5f-b105-f3955aeb803c%2Fc9a11e43-1c63-4a95-b4d3-c779dac88a8c%2F5vh76dm_processed.jpeg&w=3840&q=75)
Transcribed Image Text:# Buffer Calculation Exercise
## Problem Statement
A hypothetical Buffer C contains 1.000 M \( \text{HC}_2\text{H}_3\text{O}_2 \) and 1.000 M \( \text{C}_2\text{H}_3\text{O}_2^- \). Answer the following questions in the text boxes provided with the correct amount of significant figures. \( K_a = 1.8 \times 10^{-5} \).
### Important Instructions
- Enter the number only (no units).
- Do not leave any spaces.
- Use a leading zero before the decimal when necessary.
- Report your numbers to the proper significant figures.
## Questions and Calculations
1. **Ratio Calculation**
\[
\frac{[\text{C}_2\text{H}_3\text{O}_2^-]}{[\text{HC}_2\text{H}_3\text{O}_2]} = \_\_\_\_\_\_\_\_
\]
2. **Initial pH Calculation**
\[
\text{pH}_{\text{initial of the buffer}} = \_\_\_\_\_\_\_\_
\]
### Buffer Titration
A 20.00 mL sample of Buffer C is combined with 5.00 mL of 0.5000 M NaOH.
3. **Initial Moles Calculation**
\[
\text{Initial moles of each conjugate (both are the same)} = \_\_\_\_\_\_\_\_ \text{ mol } \text{C}_2\text{H}_3\text{O}_2^- \text{ or } \text{HC}_2\text{H}_3\text{O}_2
\]
4. **Moles Converted Calculation**
\[
\text{Moles converted by the NaOH} = \_\_\_\_\_\_\_\_ \text{ mol } \text{C}_2\text{H}_3\text{O}_2^- \text{ or } \text{HC}_2\text{H}_3\text{O}_2
\]
5. **Total Volume Calculation**
\[
\text{Total volume} = \_\_\_\_\_\_\_\_ \text{ L}
\]
Expert Solution
Step 1
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY