a Choose the correct structure for the following compounds. a-D-Glucose-1-phosphate CH2OH о-Р-ОН ОН OH OH OH CH2OH OH Но-Р-О ÓH ÓH OH O=P-OH CH2O OH ÓH ÓH ÓH CH2OH OH ÓH ÓH О-Р-ОН ÓH CH,OH
a Choose the correct structure for the following compounds. a-D-Glucose-1-phosphate CH2OH о-Р-ОН ОН OH OH OH CH2OH OH Но-Р-О ÓH ÓH OH O=P-OH CH2O OH ÓH ÓH ÓH CH2OH OH ÓH ÓH О-Р-ОН ÓH CH,OH
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question

Transcribed Image Text:**Question:**
Choose the correct structure for the following compounds.
**α-D-Glucose-1-phosphate**
- Option 1: Structure with a six-membered ring, including one oxygen atom. The glucose molecule has a phosphate group (O=P-OH) attached at position 1.
- Option 2: Similar six-membered ring structure with a different arrangement. The phosphate group is shown as HO-O-P=O.
- Option 3: Another six-membered ring with a phosphate group attached at position 1. The phosphate is shown as O=P-OH, with the OH group oriented differently.
- Option 4: Six-membered ring structure with the phosphate group (O=P-OH) attached at position 1 in a reversed orientation.
- Option 5: Same base ring structure without a phosphate group attached in the main representation.

Transcribed Image Text:The image depicts four chemical structures of sugar phosphates. Here is a detailed explanation:
1. **First Structure**:
- A cyclic sugar ring, likely in a furanose or pyranose form.
- Attached to the ring is a phosphate group (\( \text{HO--P(=O)(OH)--O} \)) linked to the 1’ carbon of the sugar.
- Hydroxyl (OH) groups are attached.
2. **Second Structure**:
- Similar cyclic sugar ring.
- A phosphate group (\( \text{O--P(=O)(OH)--O} \)) is linked to the 6’ position via a CH\(_2\)O group.
- Other hydroxyl (OH) groups are attached to the carbon ring.
3. **Third Structure**:
- Another cyclic sugar structure.
- Features a CH\(_2\)OH group linked to the 6’ position.
- A phosphate group (\( \text{O--P(=O)(OH)--OH} \)) is directly linked to the 3’ position.
4. **Fourth Structure**:
- Also a ringed sugar form.
- Exhibits a CH\(_2\)OH group connected to the 6’ position.
- A phosphate group (\( \text{O--P(=O)(OH)--OH} \)) attached at the 5’ position.
- Hydroxyl (OH) groups are also present.
Each structure is presented as part of a list, indicated by circular bullets. These diagrams represent variations of phosphorylated sugars, commonly involved in biochemical pathways like glycolysis.
Expert Solution
Step 1
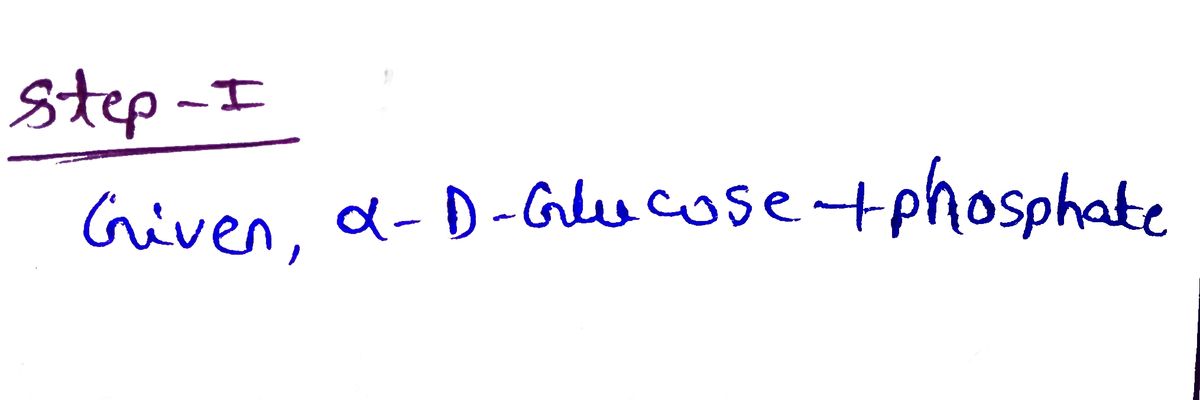
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY