42. Describe the Lyman, Ballmer and Paschen series
Related questions
Question

Transcribed Image Text:42. Describe the Lyman, Ballmer and Paschen series
Expert Solution
Step 1
When an electron is excited to an outer orbit, it try to get to its old or lower orbits.
Therefore electron comes from higher orbit to lower orbit and the difference of equvaley energy released in the form of photon or spectrum lines.
These lines are mainly six groups in the hydrogen atomic spectrum. These are following
1-Lyman series
2- Ballmer series
3- Paschen series
4- Brackett series
5-pfund series
6-Humphrey series
The required formula for this corresponding wavelength is given as following.
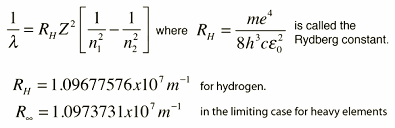
Step by step
Solved in 3 steps with 1 images
