3) At 448°C the equilibrium constant Kc for the reaction given below is 50. If we start with 100M HI, 5M H2, and 1OM of Iz in a 100 L container, H2(g) + I2(g) = 2 HI(g) AH=120 kJ a) Determine reaction quotient. b) What is the equilibrium partial pressure for each compound?
Ionic Equilibrium
Chemical equilibrium and ionic equilibrium are two major concepts in chemistry. Ionic equilibrium deals with the equilibrium involved in an ionization process while chemical equilibrium deals with the equilibrium during a chemical change. Ionic equilibrium is established between the ions and unionized species in a system. Understanding the concept of ionic equilibrium is very important to answer the questions related to certain chemical reactions in chemistry.
Arrhenius Acid
Arrhenius acid act as a good electrolyte as it dissociates to its respective ions in the aqueous solutions. Keeping it similar to the general acid properties, Arrhenius acid also neutralizes bases and turns litmus paper into red.
Bronsted Lowry Base In Inorganic Chemistry
Bronsted-Lowry base in inorganic chemistry is any chemical substance that can accept a proton from the other chemical substance it is reacting with.
can you help the question?

In any reaction, at specific time, the engaged reactant's/product's quantities estimation gets accomplished by their reaction quotient (Q). At equilibrium, Q becomes identical with K that is equilibrium constant.
Given
The equilibrium constant Kc is 50.
The temperature is 448oC.
The concentration of H2 is 5 M.
The concentration of i2 is 10 M.
The concentration of HI is 100 M.
The volume is 100 L.
Part (a):
The given reaction is as follows,
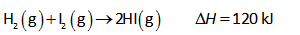
The expression for reaction quotient is shown below.
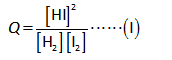
Here,
The reaction quotient is “Q”.
Substitute the known values in the equation (I).
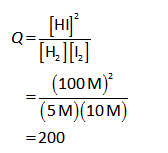
Hence, the reaction quotient of the reaction is 200.
Part (b):
The equilibrium constant expression is shown below.
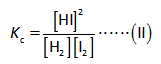
Here,
The equilibrium constant is “Kc”.
Since the concentration of product is very large in the reaction. Therefore, the reaction will proceed to the backward direction. And the concentration of reactants will increase in the reaction.
The ICE table for the given reaction is shown below.
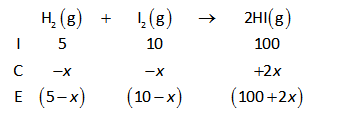
Now, substitute the known values in equation (II).
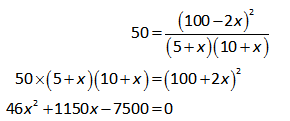
On further solving the above equation,
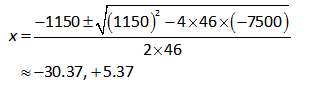
Since, the change in concentration cannot be negative.
Therefore, the value of x is 5.37M.
Step by step
Solved in 9 steps with 15 images









