2) Write the products of the following reactions and indicate if they are meso compounds or racemic mixtures. + KMNO4, KOH, cold Ph Ph Ph H, 1 atm. RT Ni (cat) Ph
2) Write the products of the following reactions and indicate if they are meso compounds or racemic mixtures. + KMNO4, KOH, cold Ph Ph Ph H, 1 atm. RT Ni (cat) Ph
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question

Transcribed Image Text:**Question 2:** Write the products of the following reactions and indicate if they are meso compounds or racemic mixtures.
**Reaction 1:**
- Starting Material: A molecule with a central carbon bonded to two phenyl (Ph) groups and two methyl groups (one extending upward and one extending downward).
- Reaction Conditions: The molecule reacts with potassium permanganate (KMnO₄) and potassium hydroxide (KOH) under cold conditions.
**Reaction 2:**
- Starting Material: A similar molecule with two phenyl groups and an unsaturated bond (indicated by a double bond).
- Reaction Conditions: The molecule undergoes hydrogenation with hydrogen gas (H₂) at 1 atmospheric pressure and room temperature (RT) in the presence of a nickel (Ni) catalyst.
Expert Solution
Step 1
Both the 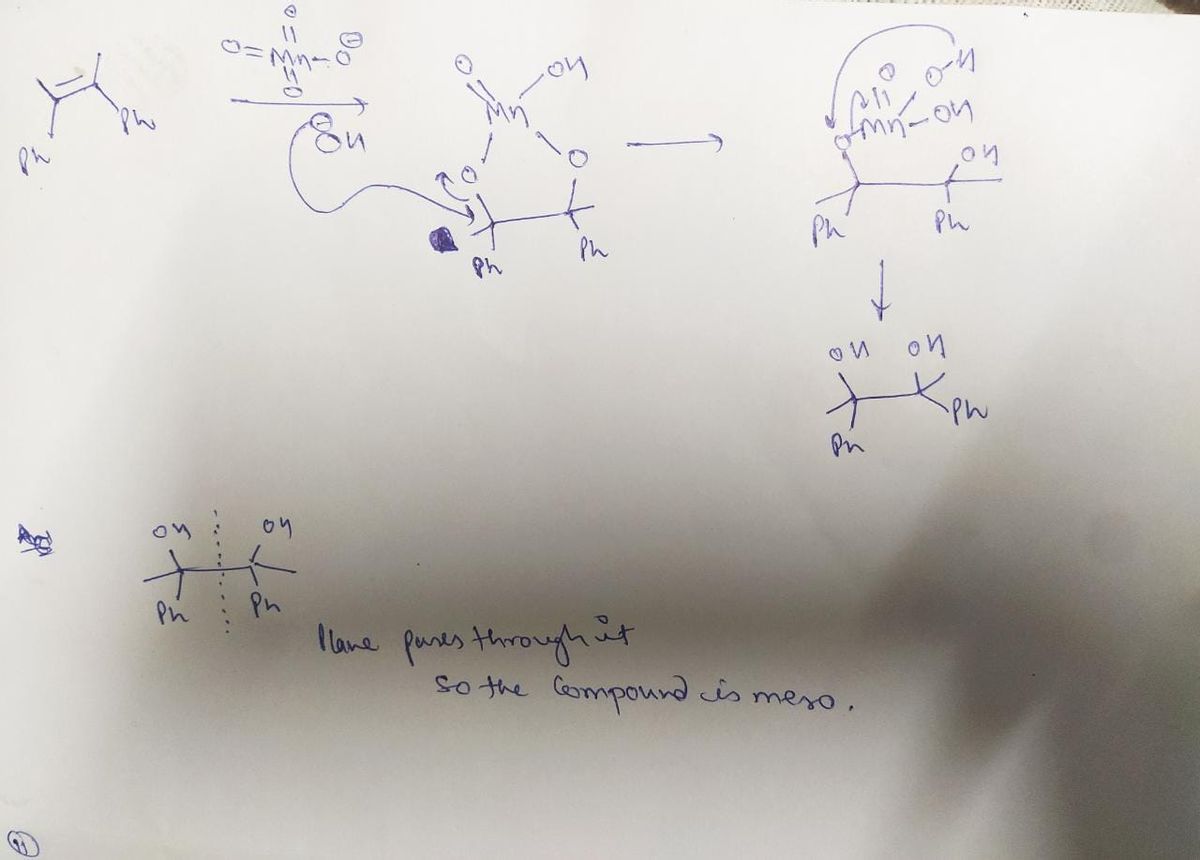 reaction give meso compound because in both reaction their should be syn addition and after syn addition plane of symmetry formed
reaction give meso compound because in both reaction their should be syn addition and after syn addition plane of symmetry formed
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY