Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
Draw the lowest energy Newman Projection for each compound.

Transcribed Image Text:### Image Transcription
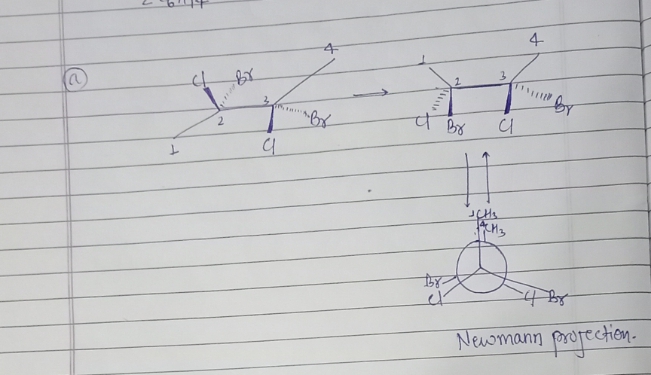
#### Diagram a
This diagram illustrates the molecular structure of a carbon chain labeled from 1 to 4. The structure is viewed along the C2-C3 axis.
- **Atoms Attached:**
- Carbon 2 is bonded to a chlorine atom (Cl) positioned as a solid wedge (indicating a bond projecting out of the plane toward the viewer) and a bromine atom (Br) as a dashed wedge (pointing into the plane away from the viewer).
- Carbon 3 is similarly bonded to a chlorine atom (Cl) positioned as a solid wedge and a bromine atom (Br) as a dashed wedge.
The solid and dashed wedges are used to illustrate stereochemistry, showing the three-dimensional orientation of the chlorine and bromine atoms in the molecule.
#### Diagram b
This diagram shows another view of a carbon chain, labeled from 1 to 5, observed along the C3-C4 axis.
- **Atoms Attached:**
- Carbon 4 is bonded to a chlorine atom (Cl) with straight lines representing bonds in the plane of the page. The other branches connected to the carbon atoms are unlabeled.
Both diagrams convey different perspectives of the molecular structure, vital for understanding stereochemistry and three-dimensional configurations in organic molecules.
Expert Solution
Step 1
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY