2) A radioactive sample reduced to 35% of its initial value in 2 hours. Calculate the half life of the sample
Q: When we talk about radioactive decay, we often talk about a half-life, i.e. the amount of time it…
A:
Q: A radioactive sample has a decay constant of 55.0 x 10-¹2 s¹. Estimate its half-life.
A: Radioactive decay is the process by which unstable nuclei spontaneously lose energy to become…
Q: 2. An isotope of sodium 24N a has a half-life of 15 hours. A sample of this isotope has a mass of…
A:
Q: After 2.14 days, the activity of a sample of an unknown type radioactive material has decreased to…
A:
Q: Determine the nuclear radius (in fm) for each of the following nuclei. 24 (a) 12 Mg fm 18 (b) fm
A:
Q: ii) The half-life of a radioactive element is 20 days. Calculate the time taken for the number of…
A: Given Data : Half life of element, t1/2 = 20 days Number of nuclei left = 40% of initial value…
Q: 3) A radioactive sample reduced to 45% of its initial value in 5 minutes. Calculate the half life of…
A: When there is amismathc between the number of protons and neutrons inside a nucleus they become…
Q: 2. Consider a radioactive substance with a half-life of H = 25 days. Suppose mo = 50 mg is the…
A:
Q: What fraction of a radioactive sample is left after exactly 6 half lives? Give your answer to 3…
A: Number of half life (n) = 6
Q: A radioactive sample is observed for 160 days. After 160 days the amount of the sample is found to…
A: Radioactive decay is the process by which an unstable nucleus becomes stable. The rate at which this…
Q: The half-life of radon-222 is about 3.823 days. (a) Determine the decay constant for radon-222. s -1…
A: Given data: The half life of randon-222 is t1/2=3.823 days=330307.2 s Part a: The expression for…
Q: Choose the BEST answer to the following. A radioactive sample has a half-life of 1 hour. Starting…
A: The number of half -life passed in a time is,
Q: A radioactive sample is observed for 5 days. After 5 days the amount of the sample is found to be…
A: Radioactive decay is the process by which an unstable nucleus becomes stable. The rate at which this…
Q: Match each illustration or description to the corresponding type of radioactive decay. Answer…
A: The emitted particle from the alpha decay has a +2 electric charge. An electron is emitted from the…
Q: 1. The decay of radioactive substance is given by -t T N = Nᵒe (1), where N is the number of atoms…
A: You are using incorrect symbol for Tau (decay constant).
Q: In a lab a radioactive sample is studied for 5 days. It was observed that the radioactive nuclides…
A: Radioactive decay is the process by which an unstable nucleus becomes stable. The decay process is…
Q: A radioactive sample studied in a lab is found to have a half-life of 48 hours. If the initial…
A: Radioactive decay is the process by which an unstable nucleus becomes stable. The half-life for a…
Q: The half-life of a radioactive isotope is known to be exactly 1 h. a) What fraction of a sample…
A: Given in question - Half-life (T) = 1 hour Solution: Step1 - Here we will understand the concept…
Q: A sample of a radioactive isotope is placed near a Geiger counter, which is observed to register 160…
A:
Q: 29) Strontium 90 decays at a constant rate of 2.44% per year. Therefore, the equation for the amount…
A:
Q: The half-life of radium is about 1600 years and its atomic number is 226. Calculate (i) the…
A: i. 95.76 %ii. 1.102*10^(15) disintegrations/yearExplanation:Step 1: For part (i), calculate the…
Q: Complete the following radioactive decay formulas: a) 12 5 B->___+e-+V b) 284 90 Th->250 88…
A: b)90284Th→88250Ra+___This equation describes an alpha decay, where an alpha particle, which is a…
Q: 8. The half-life for a radioactive isotope is 4.00 × 10⁹ yr. Determine the age (in years) of a rock…
A:
Q: A radioactive sample is prepared; 10.78 hours later, only 1/16th of the original sample remains.…
A: Activity N=N0e-λt 10.78 hours later, only 1/16th of the original sample remains N016=N0e-λ10.78…
Q: A 10 g sample of a radioactive element took 12 days to reduce into 1 g. Calculate the half-life and…
A: Some configurations of protons and neutrons inside a nucleus are unstable. When an atomic nucleus is…
Q: 1) A radioactive sample reduced to 10% of its initial value in 10 hours. Calculate the half life of…
A: Radioactive decay is the process by which an unstable nucleus attains stability by releasing…
Q: A radioactive sample studied in a lab is found to have a half-life of 25 hours. If the initial…
A: Radioactive decay is the process by which an unstable atomic nucleus spontaneously emits particles…
Q: A radioactive sample has a half-life of 28 mins. If the initial amount of sample was 2 g, estimate…
A: Radioactive decay takes place when an atomic nucleus becomes unstable. The rate of decay is known as…
Q: 6) A sealed box was found and contained an alloy composed of equal parts by same weight of two…
A: Let No be the initial amount of each metal and Y be its age. Thus, the no. of half-lives is given…

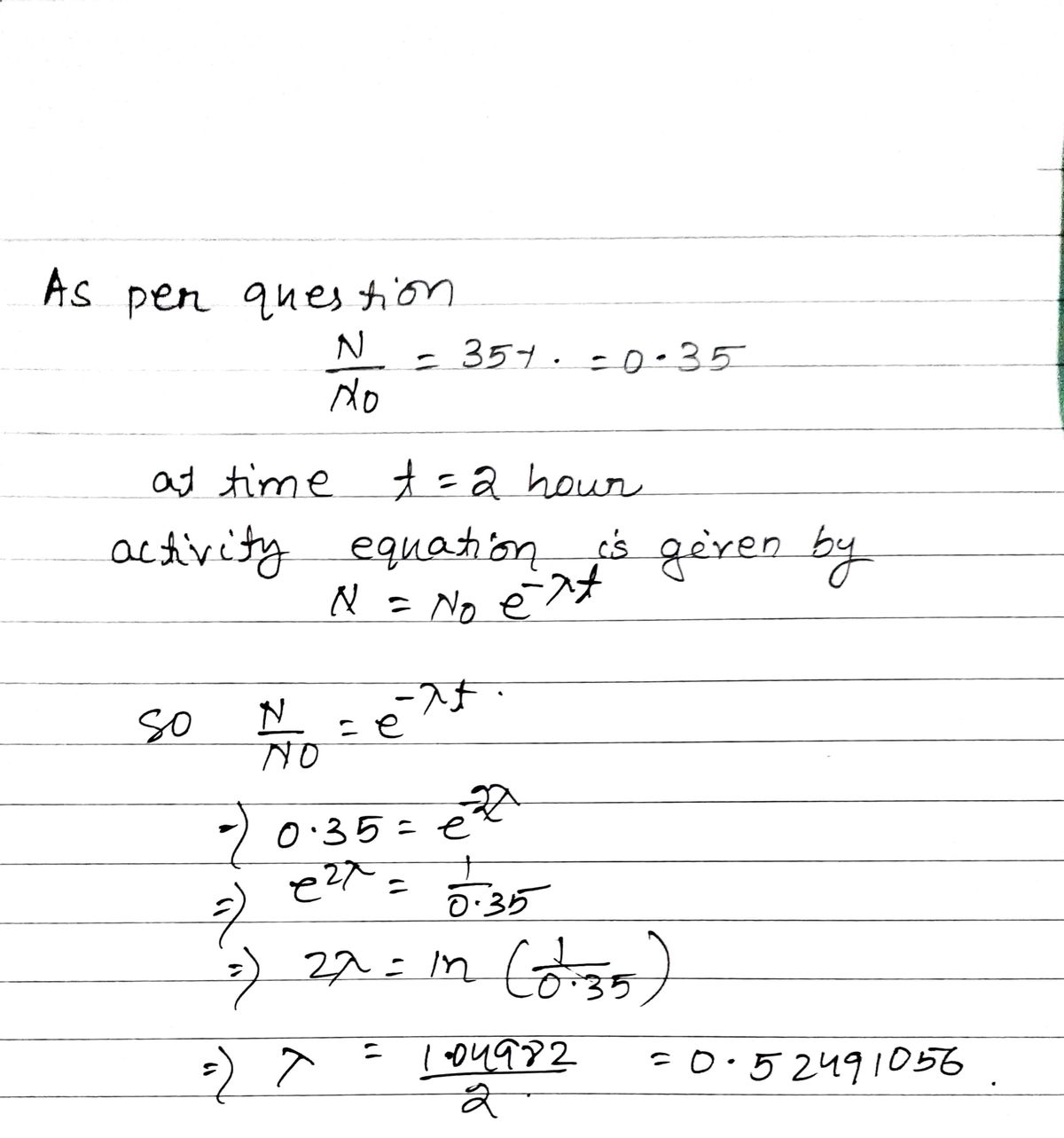
Step by step
Solved in 2 steps with 2 images

- A certain isotope has a half-life of 5.9 h and an atomic mass of 95.98 u. What will the activity in Bq of a 1.36-g sample be after 19.8 h?After 2.14 days, the activity of a sample of an unknown type radioactive material has decreased to 82.2% of the initial activity. What is the half-life of this material? days Need Help? Read ItBarium-133 is a radioactive element used in certain medical procedures. A sample of pure Barium133 has a mass of 18.000 grams. Determine the activity of the sample 47 years after it was created, in units of Curies.
- A radioactive sample has a decay constant of 49.15x10-12 s¹. Estimate the half-life.A radioactive sample is studied for 100 days. It was found that after the given period the radioactive sample reduced to 83 % of the initial amount. From the given data estimate the half-life of the sample.A sample of radioactive material has a half-life of 12 hours. If the initial sample contained 6.2x10¹3 radioactive nuclides estimate the number of radioactive nuclides present in the sample after 5 hours.
- The half-life of radon-222 is about 3.823 days. (a) Determine the decay constant for radon-222. 2.1x10-65-¹ ✓ (b) What percentage of a sample remains undecayed after two weeks (1.21 x 106 s)? %Bq. Determine the half-life (in s) of a radioactive sample that contains 1.50 x 1015 atoms and has an activity of 6.60 x 101If 88 (atomic number) 228 (atomic mass) ?? is produced, determine the parent isotope for each of the following types of decay and justify your answer. a) alpha decay b) beta negative decay c) beta positive decay