Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
Predict the approximate chemical shift of the indicated peaks in the C-NMR spectrum.

Transcribed Image Text:**13.4 Predict the approximate chemical shift of the indicated peak(s) in the \( ^{13}C \) NMR spectrum.**
**Chemical Structure:** The image shows a structural diagram of a compound with a phenyl group (benzene ring) attached to a carbonyl group (C=O) on one side and an alcohol group (OH) on the other. An arrow indicates the specific carbon atom connected to the phenyl group and the alcohol group, suggesting it is the subject of the chemical shift prediction.
**Explanation:** In the \( ^{13}C \) NMR spectrum, each type of carbon atom in a molecule produces a distinct peak. The chemical shift, measured in parts per million (ppm), reflects the electronic environment of the carbon atom. In the structure displayed:
- **Highlighted Carbon:** The carbon atom in question, attached to the oxygen-bearing substituent (OH) and the aromatic ring, will show a specific chemical shift influenced by these groups. Typically, carbon atoms adjacent to oxygen or in aromatic environments will experience downfield shifts.
**Prediction Considerations:**
- **Carbons in Aromatic Rings:** Generally appear in the range of 120-150 ppm due to the delocalized π-electron system.
- **Carbons Adjacent to Oxygen (Alcohols):** Experience deshielding and can appear around 50-80 ppm.
Based on these typical characteristics, the indicated carbon likely lies within a unique region influenced by both aromatic and oxygen such that its shift will adjust according to these neighboring electron environments.
Expert Solution
Step 1
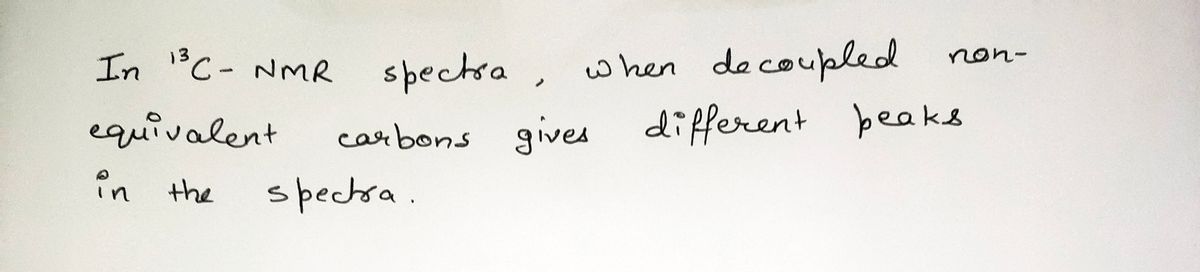
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY