Can I get some help on these.

The decay process involves the breaking of a heavier nucleus to furnish one or more daughter nuclei with the emission of radiation. The notable decay process is alpha decay, beta decay, gamma decay, and positron emission. The result of alpha decay involves the reduction in the atomic number (Z) by two and mass number (A) by four of the daughter nuclei. The beta decay involves the increase in the atomic number of the daughter nuclei. There is no change in the Z and A values of the parent and daughter nuclei in gamma decay. The positron emission involves a decrease in the Z value of the daughter nuclei by one unit.
10) The alpha decay of the americium-241 involves the formation of neptunium-237.
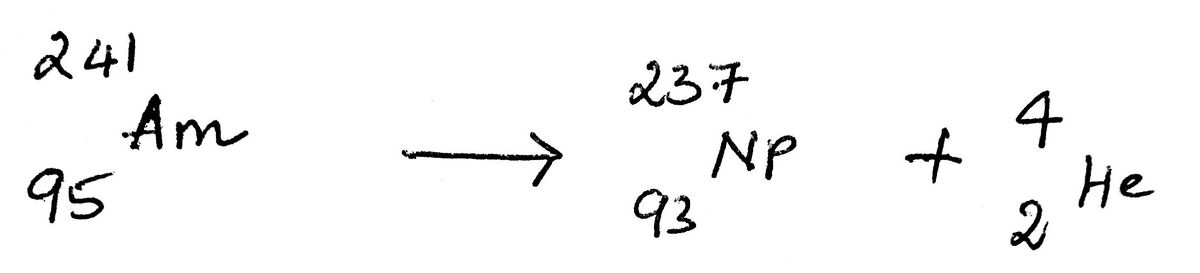
11) The beta decay of Cs-137 yields Barium-137
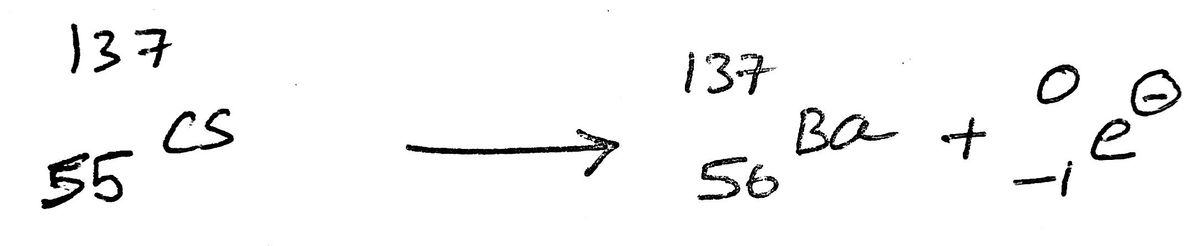
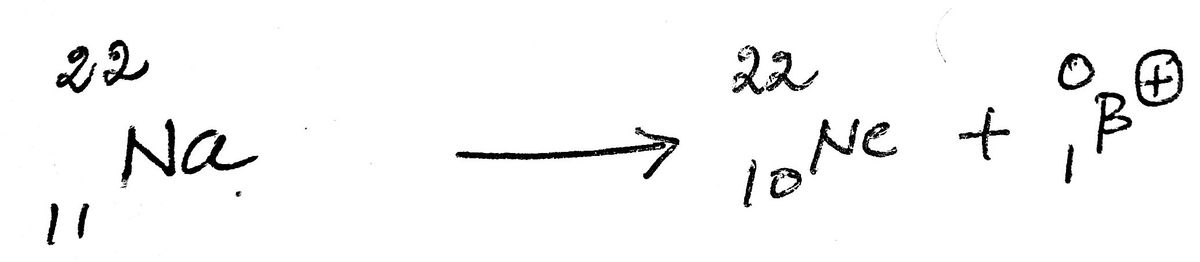
12) The positron emission of the sodium-22 provides neon-22
Step by step
Solved in 3 steps with 3 images









