
Chemistry: Structure and Properties (2nd Edition)
2nd Edition
ISBN: 9780134293936
Author: Nivaldo J. Tro
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter E, Problem 27E
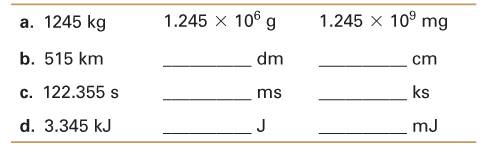
Complete the table.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
2.
Add the following group of numbers using the correct number of significant figures for the
answer. Show work to earn full credit such as rounding off the answer to the correct number
of significant figures. Replace the question marks with the calculated answers or write
the calculated answers near the question marks.
10916.345
37.40832
5.4043
3.94
+
0.0426
?
(7 significant figures)
The emf at 25°C of the cell: Pt l H2(g) l dis X:KCl (sat) l Hg2Cl2(s) l Hg l Pt was 612 mV. When solution X was replaced by normal phosphate buffer solution with a pH of 6.86, the emf was 741 mV. Calculate the pH of solution X.
Indicate how to calculate the potential E of the reaction Hg2Cl2(s) + 2e ⇄ 2Hg + 2Cl- as a function of the concentration of Cl- ions. Data: the solubility product of Hg2Cl2.
Chapter E Solutions
Chemistry: Structure and Properties (2nd Edition)
Ch. E - What are the standard SI base units of length,...Ch. E - What are the three common temperature scales? Does...Ch. E - What are prefix multipliers? List some examples.Ch. E - What is a derived unit? List an example.Ch. E - Explain the relationship between the reliability...Ch. E - What is the significance of the number of digits...Ch. E - Explain the difference between precision and...Ch. E - Prob. 8ECh. E - When multiplying or dividing measured quantities,...Ch. E - When adding or subtracting measured quantities,...
Ch. E - Explain the difference between density and mass.Ch. E - Explain the difference between intensive and...Ch. E - Prob. 13ECh. E - Prob. 14ECh. E - Prob. 15ECh. E - Prob. 16ECh. E - What is dimensional analysis?Ch. E - How should units be treated in calculations?Ch. E - Convert each temperature. a. 32 °F to °C...Ch. E - Convert each temperature. a. 212 °F to °C...Ch. E - The coldest temperature ever measured in the...Ch. E - Prob. 22ECh. E - Use the prefix multipliers to express each...Ch. E - Use prefix multipliers to express each measurement...Ch. E - Use scientific notation to express each quantity...Ch. E - Use scientific notation to express each quantity...Ch. E - Complete the table.Ch. E - Complete the table.Ch. E - Express the quantity 254,998 m in each unit. a. km...Ch. E - Express the quantity 556.2 × 10-12 s in each unit....Ch. E - How many 1-cm squares does it take to construct a...Ch. E - How many 1-cm cubes does it take to construct a...Ch. E - Convert 15.0 L to each unit. a.mL b. cm3 c. gal d....Ch. E - Convert 4.58 x 103 cm3 to each unit. a. L b. mL c....Ch. E - A ruler used to measure a penny has markings every...Ch. E - A scale used to weigh produce at a market has...Ch. E - Read each measurement to the correct number of...Ch. E - Read each measurement to the correct number of...Ch. E - For each number, underline the zeroes that are...Ch. E - For each number, underline the zeroes that are...Ch. E - How many significant figures are in each number?...Ch. E - How many significant figures are in each number?...Ch. E - Which numbers are exact (and therefore have an...Ch. E - Indicate the number of significant figures in each...Ch. E - Round each number to four significant figures. a....Ch. E - Round each number to three significant figures. a....Ch. E - Calculate to the correct number of significant...Ch. E - Calculate to the correct number of significant...Ch. E - Calculate to the correct number of significant...Ch. E - Calculate to the correct number of significant...Ch. E - Calculate to the correct number of significant...Ch. E - Calculate to the correct number of significant...Ch. E - A new penny has a mass of 2.49 g and a volume of...Ch. E - A titanium bicycle frame displaces 0.314 L of...Ch. E - Glycerol is a syrupy liquid used in cosmetics and...Ch. E - An allegedly gold nugget is tested to determine...Ch. E - Ethylene glycol (antifreeze) has a density of 1.11...Ch. E - Prob. 58ECh. E - A small airplane takes on 245 L of fuel, If the...Ch. E - Human fat has a density of 0.918 g/cm3. How much...Ch. E - Perform each unit conversion. a. 27.8 L to cm3 b....Ch. E - Prob. 62ECh. E - Prob. 63ECh. E - Prob. 64ECh. E - A runner wants to run 10.0 km. She knows that her...Ch. E - Prob. 66ECh. E - A European automobile has a gas mileage of 17...Ch. E - A gas can holds 5.0 gallons of gasoline. Express...Ch. E - A house has an area of 195 m2. What is its area...Ch. E - Prob. 70ECh. E - The average U.S. farm occupies 435 acres. (1 acre...Ch. E - Total U.S. farmland occupies 954 million acres....Ch. E - An acetaminophen suspension for infants contains...Ch. E - An ibuprofen suspension for infants contains 100...Ch. E - Convert between energy units. a. 534 kWh to J b....Ch. E - Prob. 76ECh. E - Suppose that a person eats 2387 Calories per day....Ch. E - A particular frost-free refrigerator uses about...Ch. E - Prob. 79ECh. E - Prob. 80ECh. E - A solid gold cylinder sits on a weight-sensitive...Ch. E - The proton has a radius of approximately 1.0 ×...Ch. E - The density of titanium is 4.51 g/cm3. What is the...Ch. E - The density of iron is 7.86 g/cm3. What is its...Ch. E - A steel cylinder has a length of 2.16 in, a radius...Ch. E - A solid aluminum sphere has a mass of 85 g. Use...Ch. E - Prob. 87ECh. E - Prob. 88ECh. E - The Toyota Prius, a hybrid electric vehicle, has a...Ch. E - The Honda Insight, a hybrid electric vehicle, has...Ch. E - The single proton that forms the nucleus of the...Ch. E - A sample of gaseous neon atoms at atmospheric...Ch. E - Prob. 93ECh. E - The world’s record in the 100-m dash is 9.58 s,...Ch. E - Table salt contains 39.33 g of sodium per 100 g of...Ch. E - Prob. 96ECh. E - A length of #8 copper wire (radius = 1.63 mm) has...Ch. E - Rolls of aluminum foil are 304 mm wide and 0.016...Ch. E - Liquid nitrogen has a density of 0.808 g/L and...Ch. E - Mercury is often used in thermometers. The mercury...Ch. E - Prob. 101ECh. E - In 1999, scientists discovered a new class of...Ch. E - Prob. 103ECh. E - Nanotechnology, the field of building ultrasmall...Ch. E - Prob. 105ECh. E - A box contains a mixture of small copper spheres...Ch. E - A cube has an edge length of 7 cm. If it is...Ch. E - Prob. 108ECh. E - For each box, examine the blocks attached to the...Ch. E - Look up the measurement of the approximate...Ch. E - Prob. 111ECh. E - One inch is equal to 2.54 cm. Draw a line that is...Ch. E - Convert the height of each member in your group...Ch. E - Prob. 114ECh. E - Convert 85.0 °F to k. 358 k 181.1 k 302.6 k 29.4 kCh. E - Express the quantity 33.2 × 10-4 m in mm. 0.332 mm...Ch. E - How many significant figures are there in the...Ch. E - Perform the calculation to the correct number of...Ch. E - Perform the calculation to the correct number of...Ch. E - What is the mass of a 1 .75-L sample of a liquid...Ch. E - Convert 1,285 cm2to m2. 12.85 m2 0.1285 m2 1.285 ×...Ch. E - Prob. 8SAQCh. E - A solid metal sphere has a radius of 3.53 cm and a...Ch. E - A German automobile’s gas mileage is 22km/L....Ch. E - A wooden block has a volume of 18.5 in3. Express...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- How can Beer’s Law be used to determine the concentration in a selected food sample. Provide an in-depth discussion and examples of this.arrow_forwardb) H3C- H3C Me CH 3 I HN Me H+arrow_forwardUsing Luther's rule, determine the reference potentials of the electrodes corresponding to the low stability systems Co³+/Co and Cr²+/Cr from the data in the table. Electrodo ΕΝ Co²+/Co Co3+/Co²+ -0,28 +1,808 Cr³+ / Cr -0,508 Cr3+ / Cr²+ -0,41arrow_forward
- The molecule PYRIDINE, 6tt electrons and is there pore aromuntre and is Assigned the Following structure contenus Since aromatk moleculey undergo electrophilic allomatic substitution, Pyridine should undergo The Following reaction + HNO3 12504 a. write all of the possible Mononitration Products that could Result From this roaction Based upon the reaction the reaction mechanism determine which of these producty would be the major Product of the hegetionarrow_forwardUsing Benzene as starting materia Show how each of the Following molecules could Ve synthesked 9. CHI d. 10450 b 0 -50311 ८ City -5034 1-0-650 e NO2arrow_forwardBA HBr of the fol 1)=MgCI 2) H₂O major NaOEt Ts Cl Py (pyridine) 1) 03 2) Me2S 1arrow_forward
- 4. Provide a clear arrow-pushing mechanism for the following reactions. Do not skip proton transfers, do not combine steps, and make sure your arrows are clear enough to be interpreted without ambiguity. a) NHBoc ⚫OBn HO. H3C CO2CH3 -OBn H3C H3C. H3C. NHBOC CI CO2CH3arrow_forwardDraw structures of the following compounds and identify their role: mCPBA (MCPBA) DMS Py 9-BBN LAH Sia₂BH TsCI PCC t-BuOK LDA MeLi n-BuLi DMSO DMF Sodium Borohydride Lithium DiisopropylAmide 2arrow_forwardUsing Luther's rule, calculate the reference potential of the Hg2+/Hg redox electrode. DATA: Electrode potentials E° = 0,854 V y E 0,788 V Hg2+/Hg 2+ Hg2/Hgarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Atomic Number, Atomic Mass, and the Atomic Structure | How to Pass ChemistryThe Nucleus: Crash Course Chemistry #1; Author: Crash Course;https://www.youtube.com/watch?v=FSyAehMdpyI;License: Standard YouTube License, CC-BY