
Concept explainers
Interpretation:
The structural formulas involved in the sequence of given reactions for the formation of adipic acid or hexamethylene diamine are to be written, with given molecular formula of each compound and IR data of some compound.
Concept introduction:
A
Nylon is a polymer that consists of adipic acid and hexamethylene diamine as monomer units.
Nylon
The formation of Nylon
The addition of hydrogen to double or triple bond is a hydrogenation reaction.
Hydrogenation of
Infrared spectroscopy is a simple, instrumental technique, which helps to determine the presence of various functional groups.
It depends on the interactions of atoms or molecules with the electromagnetic radiation.
The molecules that have dipole moment are IR active and the molecules that do not have dipole moment are IR inactive.
Cyclohexanol shows IR peak at
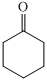
Cyclic ketone shows IR peak at
Answer to Problem 1PP
Solution:
(a) The structures involved in the sequence are as follows:
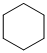 ,
, 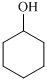 ,
, 
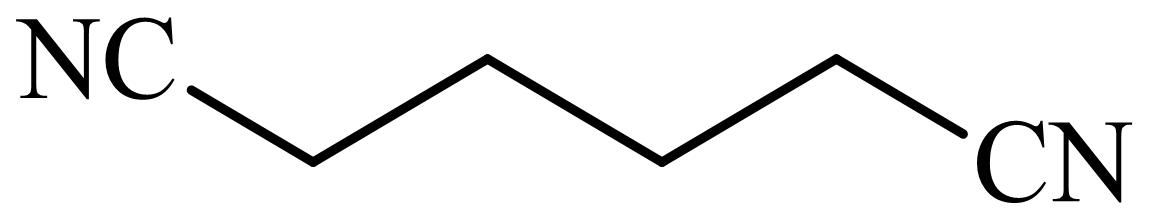
(b) The structures involved in the sequence are as follows:
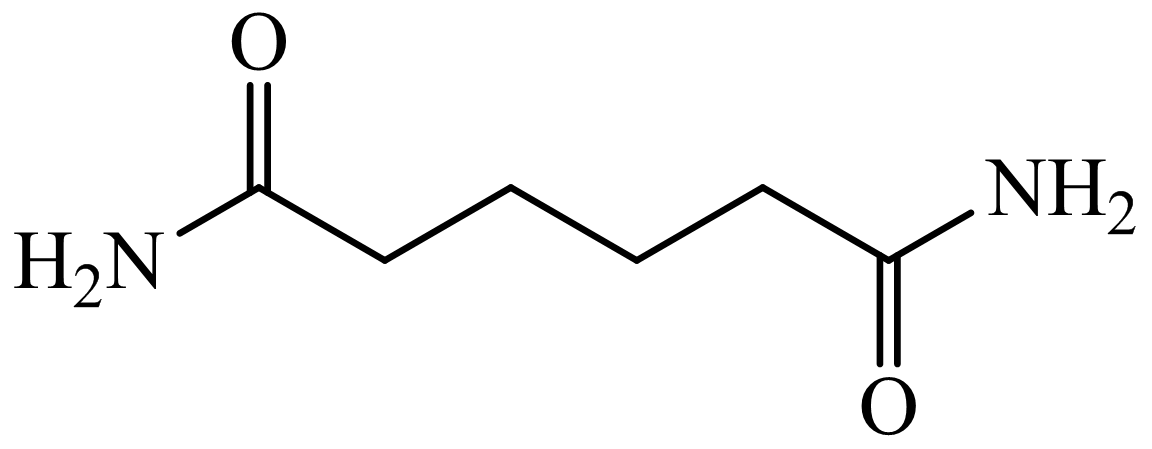 ,
, 
(c) The structures involved in the sequence are as follows:
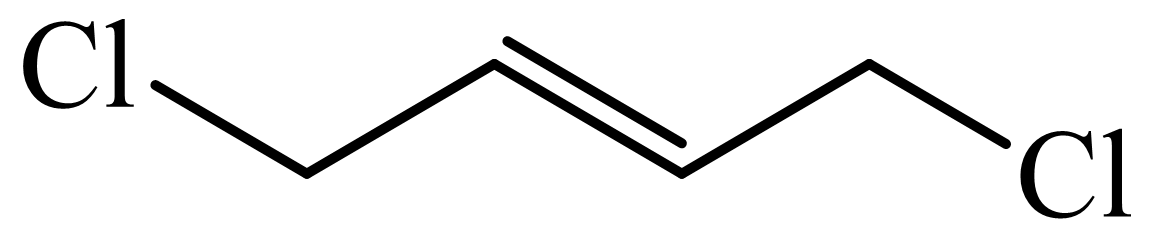 ,
, 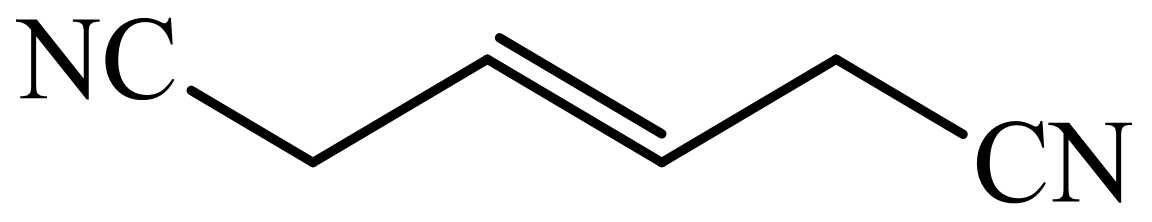 ,
,
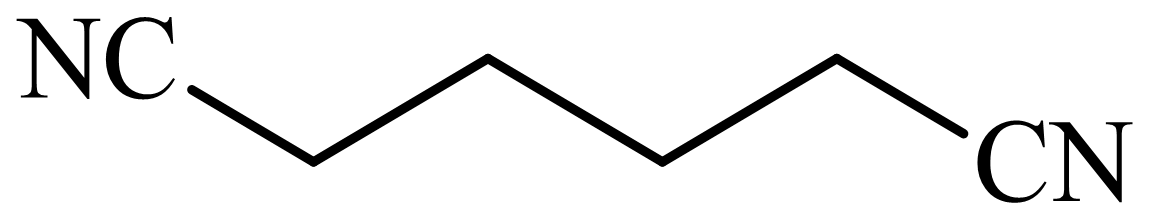
(d) The structures involved in the sequence are as follows:
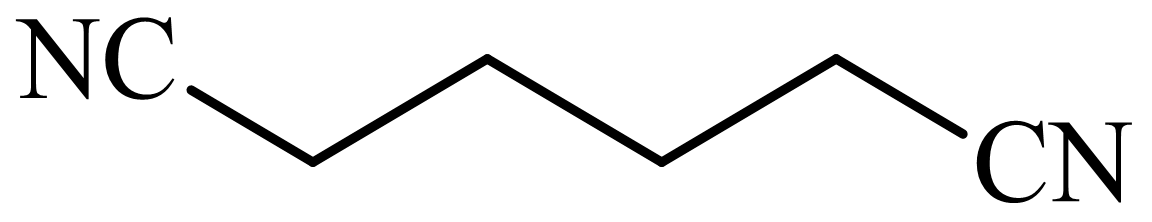
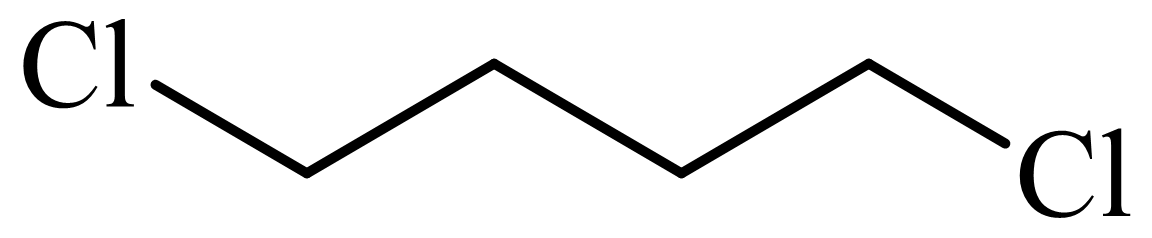 ,
, 
Explanation of Solution
a)
For the formation of adipic acid, first, benzene undergoes hydrogenation reaction to form cyclohexane, which then undergoes oxidation in the presence of catalyst to give cyclohexanol ( I.R. peak at
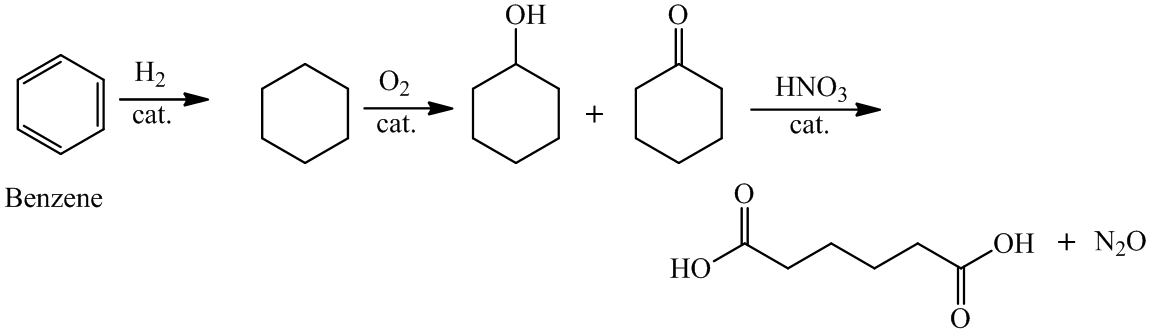
Hence, adipic acid is formed in this sequence of reactions.
b)
Here, adipic acid reacts with two molecules of ammonia to form ammonium salt of adipic acid. Then, on heating, this salt loses two molecules of water to form
The whole sequence can be written as
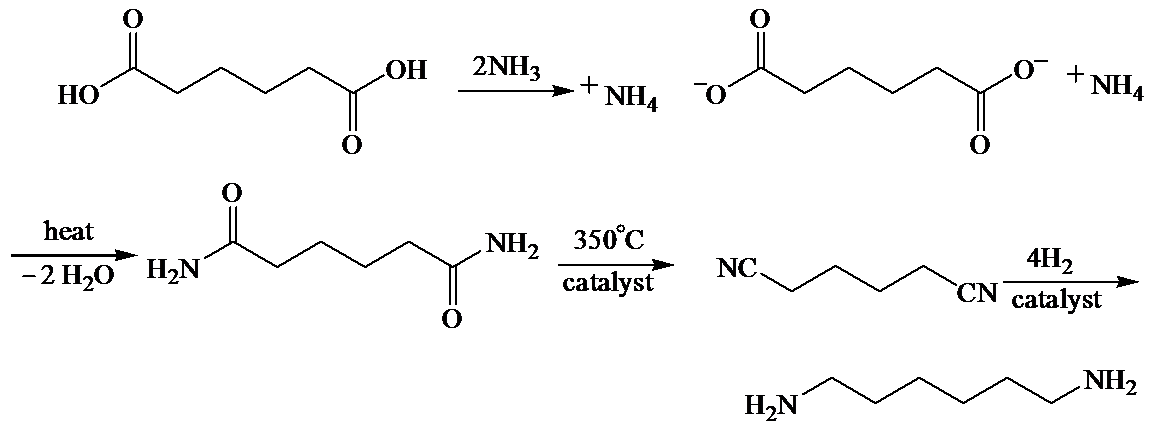
Hence, hexamethylene diamine is formed in this sequence of reactions.
c)
Here,
The whole sequence can be written as
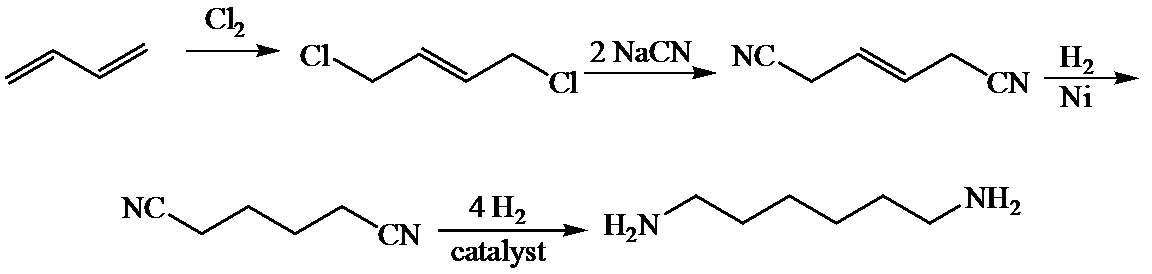
Hence, hexamethylene diamine is formed in this sequence of reactions.
d)
Here, tetrahydrofuran undergoes chlorination in the presence of hydrochloric acid resulting in the formation of
The whole sequence can be written as
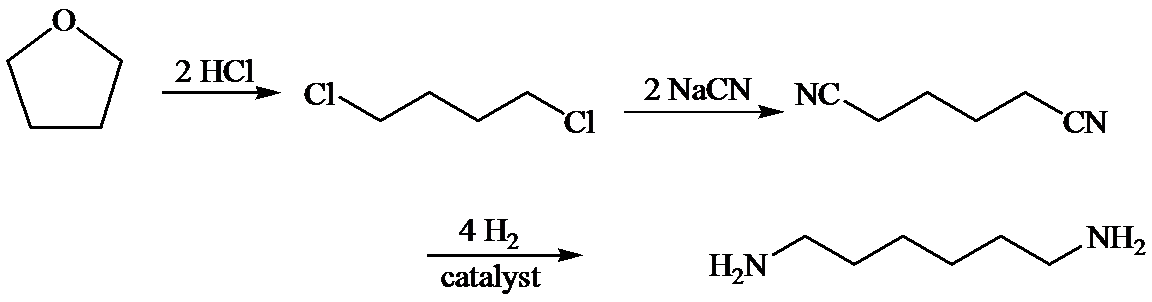
Hence, hexamethylene diamine is formed in this sequence of reactions.
Want to see more full solutions like this?
Chapter E Solutions
ORGANIC CHEM. VOL.1+2-W/WILEYPLUS
- K Draw the starting structure that would lead to the major product shown under the provided conditions. Drawing 1. NaNH2 2. PhCH2Br 4 57°F Sunny Q Searcharrow_forward7 Draw the starting alkyl bromide that would produce this alkyne under these conditions. F Drawing 1. NaNH2, A 2. H3O+ £ 4 Temps to rise Tomorrow Q Search H2arrow_forward7 Comment on the general features of the predicted (extremely simplified) ¹H- NMR spectrum of lycopene that is provided below. 00 6 57 PPM 3 2 1 0arrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

