
Concept explainers
Practice Problem C.1
(a) Write structural formulas for portions of the chain of the atactic, syndiotactic, and iso-tactic forms of polystyrene (see Practice Problem 10.15). (b) If solutions were made of each of these forms of polystyrene, which solutions would you expect to show optical activity?
Interpretation:
The structural formula for the atactic, syndiotactic, and isotactic forms of polystyrene is to be determined.
Concept introduction:
>The polymer, on the basis of stereochemistry around the chiral center, is classified as atactic, syndiotactic, and isotactic.
>A polymer in which the stereochemistry at the chiral centre is random is said to be the atactic polymer.
>A polymer in which the stereochemistry at the chiral centre alternates regularly, from one side to the other, on the chain is said to be the syndiotactic polymer.
>A polymer in which the stereochemistry of all chiral centres is the same is said to be the isotactic polymer.
>The polystyrene is the aromatic hydrocarbon polymer of the monomer styrene.
>A molecule is considered optically-active if it contains an achiral center and its mirror image is non-superimposable.
>The molecules which are non-superimposable or not identical with their mirror images are known as chiral molecules.
>A pair of two mirror images which are non-identical is known as enantiomers which are optically active.
>The objects or molecules which are superimposable with their mirror images are achiral objects or molecules and these objects have a centre of symmetry or plane of symmetry.
>The achiral compounds in which plane of symmetry is present internally and consists of chiral centres are known as meso compounds, but they are optically inactive.
>Diastereomers are the stereoisomers that are not mirror images of each other and are not superimposable on each other.
>They possess different physical as well as chemical properties, because of difference in orientations.
Answer to Problem 1PP
Solution:
(a) The structural formula for atactic, syndiotactic, and isotactic forms of polystyrene is as:
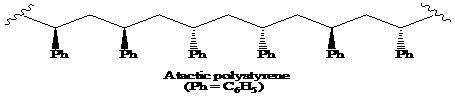
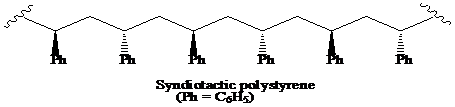
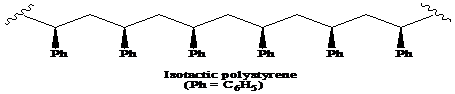
(b) The solution of isotactic polystyrene shows optical activity.
Explanation of Solution
a) The structural formula for portion of the chain of the atactic, and iso-tactic forms of polystyrene.
On the basis of arrangement of substituents on the chiral centre in the chain, the polymer is classified into atactic, syndiotactic, and isotactic.
A polymer in which the stereochemistry at the chiral centre is random is said to be the atactic polymer.
A polymer in which the stereochemistry at the chiral centre alternates regularly, from one side to the other, on the chain is said to be the syndiotactic polymer.
A polymer in which the stereochemistry of all chiral centres is the same is said to be the isotactic polymer.
Thus, the structural formula for the atactic form of polystyrene is as:
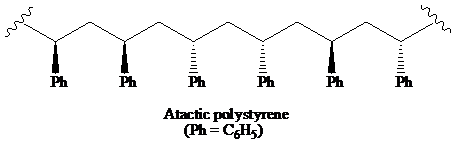
Thus, the structural formula for the syndiotactic form of polystyrene is as:

Thus, the structural formula for the isotactic form of polystyrene is as:
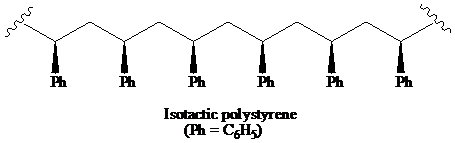
b) The solution that expected to show optical activity.
A molecule is considered optically-active if it contains an achiral center and its mirror image is non-superimposable. Among the solutions of atactic, syndiotactic, and isotactic forms of polystyrene, the solution of isotactic polystyrene rotates the plane-polarized light and its mirror image is non-superimposable. Thus, the isotactic polystyrene shows optical activity.
Therefore, the solution of isotactic polystyrene shows optical activity.
Want to see more full solutions like this?
Chapter C Solutions
Organic Chemistry
Additional Science Textbook Solutions
College Physics: A Strategic Approach (3rd Edition)
Microbiology: An Introduction
Campbell Biology (11th Edition)
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Human Biology: Concepts and Current Issues (8th Edition)
- Select the stronger base from each pair of compounds. (a) H₂CNH₂ or EtzN (b) CI or NH2 NH2 (c) .Q or EtzN (d) or (e) N or (f) H or Harrow_forward4. Provide a clear arrow-pushing mechanism for each of the following reactions. Do not skip proton transfers, do not combine steps, and make sure your arrows are clear enough to be interpreted without ambiguity. a. 2. 1. LDA 3. H3O+ HOarrow_forwardb. H3C CH3 H3O+ ✓ H OHarrow_forward
- 2. Provide reagents/conditions to accomplish the following syntheses. More than one step is required in some cases. a. CH3arrow_forwardIdentify and provide an explanation that distinguishes a qualitative and quantitative chemical analysis. Provide examples.arrow_forwardIdentify and provide an explanation of the operational principles behind a Atomic Absorption Spectrometer (AAS). List the steps involved.arrow_forward
- Instructions: Complete the questions in the space provided. Show all your work 1. You are trying to determine the rate law expression for a reaction that you are completing at 25°C. You measure the initial reaction rate and the starting concentrations of the reactions for 4 trials. BrO³¯ (aq) + 5Br¯ (aq) + 6H* (aq) → 3Br₂ (l) + 3H2O (l) Initial rate Trial [BrO3] [H*] [Br] (mol/L) (mol/L) | (mol/L) (mol/L.s) 1 0.10 0.10 0.10 8.0 2 0.20 0.10 0.10 16 3 0.10 0.20 0.10 16 4 0.10 0.10 0.20 32 a. Based on the above data what is the rate law expression? b. Solve for the value of k (make sure to include proper units) 2. The proposed reaction mechanism is as follows: i. ii. BrО¸¯ (aq) + H+ (aq) → HBrO3 (aq) HBrO³ (aq) + H* (aq) → H₂BrO3* (aq) iii. H₂BrO³* (aq) + Br¯ (aq) → Br₂O₂ (aq) + H2O (l) [Fast] [Medium] [Slow] iv. Br₂O₂ (aq) + 4H*(aq) + 4Br(aq) → 3Br₂ (l) + H2O (l) [Fast] Evaluate the validity of this proposed reaction. Justify your answer.arrow_forwardе. Д CH3 D*, D20arrow_forwardC. NaOMe, Br Brarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





