
Concept explainers
(a)
Interpretation:
The IUPAC name for the given compound is to be determined.
Concept introduction:
In case of molecules containing a
If the root is a chain, numbering begins from that end of the chain which encounters the
The carbon atoms having a double or triple bond between them are always assigned C1 and C2, if the root is a ring. This must be done such that the locator numbers for the substituents are minimized. The lower of the two locator numbers for the
Answer to Problem B.23P
The IUPAC name for the given compound is:
Explanation of Solution
The given molecule is:
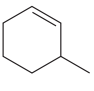
In this molecule, the largest carbon ring containing the double bond has six carbon atoms. Hence, the root is cyclohexene. The ring is numbered such that the double bonded carbon atoms get C1 and C2 as the locator numbers.
The numbering system is shown below:
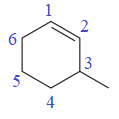
One methyl group is attached at the C3 carbon atom of the ring.
Thus, the IUPAC name of the compound is:
The IUPAC name of the compound is written according to the rules for nomenclature.
(b)
Interpretation:
The IUPAC name for the given compound is to be determined.
Concept introduction:
In case of molecules containing a
If the root is a chain, numbering begins from that end of the chain which encounters the
The carbon atoms having a double or triple bond between them are always assigned C1 and C2, if the root is a ring. This must be done such that the locator numbers for the substituents are minimized. The lower of the two locator numbers for the
Answer to Problem B.23P
The IUPAC name for the given compound is:
Explanation of Solution
The given compound is:
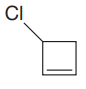
In this molecule, the largest carbon ring containing the double bond has four carbon atoms. Hence, the root is cyclobutene. The ring is numbered such that the double bonded carbon atoms get C1 and C2 as the locator numbers.
The numbering system is shown below:
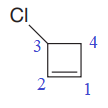
One chlorine atom is attached as a substituent on the C3 carbon atom of the ring.
Thus, the IUPAC name of the compound is:
The IUPAC name of the compound is written according to the rules for nomenclature.
(c)
Interpretation:
The IUPAC name for the given compound is to be determined.
Concept introduction:
In case of molecules containing a
If the root is a chain, numbering begins from that end of the chain which encounters the
The carbon atoms having a double or triple bond between them are always assigned C1 and C2, if the root is a ring. This must be done such that the locator numbers for the substituents are minimized. The lower of the two locator numbers for the
Answer to Problem B.23P
The IUPAC name for the given compound is:
Explanation of Solution
The given molecule is:
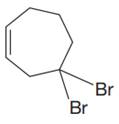
In this molecule, the largest carbon ring containing the double bond has seven carbon atoms. Hence, the root is cycloheptene. The ring is numbered such that the double bonded carbon atoms get C1 and C2 as the locator numbers.
The numbering system is shown below:
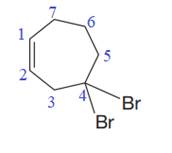
Two bromine atoms are attached to the C4 carbon atom of the ring. Thus, a prefix ‘di’ must be used to indicate the number of bromide substituents.
Thus, the IUPAC name of the compound is:
The IUPAC name of the compound is written according to the rules for nomenclature.
(d)
Interpretation:
The IUPAC name for the given compound is to be determined.
Concept introduction:
In case of molecules containing a
If the root is a chain, numbering begins from that end of the chain which encounters the
The carbon atoms having a double or triple bond between them are always assigned C1 and C2, if the root is a ring. This must be done such that the locator numbers for the substituents are minimized. The lower of the two locator numbers for the
Answer to Problem B.23P
The IUPAC name for the given compound is
Explanation of Solution
The given molecule is:
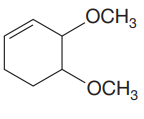
In this molecule, the largest carbon ring containing the double bond has six carbon atoms. Hence, the root is cyclohexene. The ring is numbered such that the double bonded carbon atoms get C1 and C2 as the locator numbers.
The numbering system is shown below:
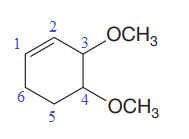
At C3 and C4 carbon atoms of the ring, two methoxy substituents are attached.
Thus, a prefix ‘di’ must be used for methoxy substituents to indicate their number.
Thus, the IUPAC name of the compound is
The IUPAC name of the compound is written according to the rules for nomenclature.
(e)
Interpretation:
The IUPAC name for the given compound is to be determined.
Concept introduction:
In case of molecules containing a
If the root is a chain, numbering begins from that end of the chain which encounters the
The carbon atoms having a double or triple bond between them are always assigned C1 and C2, if the root is a ring. This must be done such that the locator numbers for the substituents are minimized. The lower of the two locator numbers for the
Answer to Problem B.23P
The IUPAC name for the given compound is
Explanation of Solution
The given molecule is:
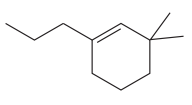
In this molecule, the largest carbon ring containing the double bond has six carbon atoms. Hence, the root is cyclohexene. The ring is numbered such that the double bonded carbon atoms get C1 and C2 as the locator numbers.
The numbering system is shown below:
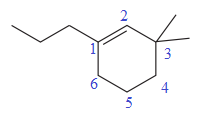
At C1 carbon atom of the ring, a substituent propyl is attached. At C3 carbon atom of the ring, two methyl groups are attached. Thus, a prefix ‘di’ must be used for methyl substituents to indicate the number. Out of propyl and methyl substituents, methyl comes first alphabetically, hence it will be written first in the name.
Thus, the IUPAC name of the compound is
The IUPAC name of the compound is written according to the rules for nomenclature.
(f)
Interpretation:
The IUPAC name for the given compound is to be determined.
Concept introduction:
In case of molecules containing a
If the root is a chain, numbering begins from that end of the chain which encounters the
The carbon atoms having a double or triple bond between them are always assigned C1 and C2, if the root is a ring. This must be done such that the locator numbers for the substituents are minimized. The lower of the two locator numbers for the
Answer to Problem B.23P
The IUPAC name for the given compound is
Explanation of Solution
The given molecule is:
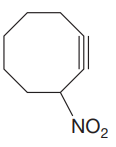
In this molecule, the largest carbon ring containing the triple bond has eight carbon atoms. Hence, the root is cyclooctayne. The ring is numbered such that the double bonded carbon atoms get C1 and C2 as the locator numbers.
The numbering system is shown below:
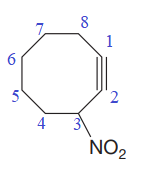
At C3 carbon atom of the ring, a substituent nitro is attached.
Thus, the IUPAC name of the compound is
The IUPAC name of the compound is written according to the rules for nomenclature.
Want to see more full solutions like this?
Chapter B Solutions
EBK GET READY FOR ORGANIC CHEMISTRY
- A mixture of C7H12O2, C9H9OCl, biphenyl and acetone was put together in a gas chromatography tube. Please decide from the GC resutls which correspond to the peak for C7,C9 and biphenyl and explain the reasoning based on GC results. Eliminate unnecessary peaks from Gas Chromatography results.arrow_forwardIs the molecule chiral, meso, or achiral? CI .CH3 H₂C CIarrow_forwardPLEASE HELP ! URGENT!arrow_forward
- Identify priority of the substituents: CH3arrow_forwardHow many chiral carbons are in the molecule? OH F CI Brarrow_forwardA mixture of three compounds Phen-A, Acet-B and Rin-C was analyzed using TLC with 1:9 ethanol: hexane as the mobile phase. The TLC plate showed three spots of R, 0.1 and 0.2 and 0.3. Which of the three compounds (Phen-A; Acet-B or Rin-C) would have the highest (Blank 1), middle (Blank 2) and lowest (Blank 3) spot respectively? 0 CH: 0 CH, 0 H.C OH H.CN OH Acet-B Rin-C phen-A A A <arrow_forward
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
