
Interpretation:
The required volume of stock solution to prepare the given volumes and concentrated final solutions are should be calculated by using given representation diagram.
Concept introduction:
Dilution:
- Dilution is the process, which is used to prepare a low concentrated solution from high concentrated solution.
Volumetric principle:
- In the dilution process, the relationship between initial and final concentrations and volumes of solutions are given in the volumetric equation and it is,
Explanation of Solution
To calculate the required volumes of stock solutions to prepare the final diluted solutions
The given diagram is,
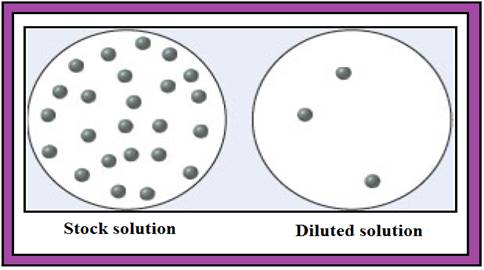
Diagram.1
To calculate the required volume of stock solution to prepare
From the given diagram (Diagram.1) the stock solution contains 24 numbers of solute particles and diluted solution contains 3 numbers of solute particles.
Consider a unit of stock solution contains 24 numbers of solute particles and a unit of diluted solution contains 3 numbers of solute particles.
The numbers of solute particles and final volume are plugged in the above equation to give required volume of stock solution to prepare
The required volume of stock solution to prepare
The required volume of stock solution to prepare
From the given diagram (Diagram.1) the stock solution contains 24 numbers of solute particles and diluted solution contains 3 numbers of solute particles.
Consider a unit of stock solution contains 24 numbers of solute particles and a unit of diluted solution contains 3 numbers of solute particles.
The numbers of solute particles and final volume are plugged in the above equation to give required volume of stock solution to prepare
The required volume of stock solution to prepare
The required volume of stock solution to prepare
From the given diagram (Diagram.1) the stock solution contains 24 numbers of solute particles and diluted solution contains 3 numbers of solute particles.
Consider a unit of stock solution contains 24 numbers of solute particles and a unit of diluted solution contains 3 numbers of solute particles.
The numbers of solute particles and final volume are plugged in the above equation to give required volume of stock solution to prepare
The required volume of stock solution to prepare
The required volume of stock solution to prepare the given volumes and concentrated final solutions are calculated by using given representation diagram.
Want to see more full solutions like this?
Chapter 9 Solutions
EBK CHEMISTRY: ATOMS FIRST
- Can you explain how I get these here and show the steps plz?arrow_forwardGive the IUPAC name for this compound Hydrocarbon Condensed Formulas Hint C2H5 CH2CH3 expand that in all the formula Part A: (CH3)2CHCH(C2H5)CH2CH2CH3 Give the IUPAC name for this compound. Part B: CH2=C(C2H5)CH2CH2CH3 Give the IUPAC name for this compound. Part C: (CH3)2C=CHC(C2H5)=CH2 Give the IUPAC name for this compound. Part D: CH3C=CCH(C2H5)2 Give the IUPAC name for this compound. Part E: (CH3)3CC=CCH2CH=C(CH3)2arrow_forwardSelect/ Match the correct letter from the image below for the IUPAC names given below: A B C D 3 E F G H K L Part 1. 4-methylheptane For example.mmmm Answer Letter H _for part 1 Part 2. 2,4-dimethylhexane Part 3. 2,3-dimethylpentane Part 4. 2,2-dimethylhexane Part 5. 2-ethyl-1,1,3,3-tetramethylcyclopentane Part 6. 3-ethyl-2-methylpentanearrow_forward
- Can u show the process as to how to get these?arrow_forwardSketch the expected 'H NMR spectra for the following compound. Label all of the H's in the structure and the corresponding signal for the spectra you sketch. Make sure you include the integration value and the splitting pattern for each signal Indicate how many signals you would expect in the 13C NMRarrow_forwardUse IUPAC naming rules to name the following hydrocarbon compounds: CH2-CH3 | a) CH-CH-CH2-CH-CH-CH3 b) | CH2 CH3 | CH3 CH3 \ / C=C H 1 H CH2-CH3 c) d) CH=C-CH3 e) CH3-CH2-CH2-CH=CH-CH3 f) CH2=CH-CH2-CH=CH-CH3 g) CH3-CH2-C = C-CH2-CH3 h)arrow_forward
- Q5 Name the following : a. b. C. d. e.arrow_forward25. Predict the major product of the following reaction. 1 equivalent of each of the starting materials was used. H₂C CH3 CH3 H3C H3C H3C. CH2 + H3C. heat CH3 CH H.C. CH3 H.C H.C CH3 CH CH3 CH3 A B C Earrow_forwardFind chemical structures based on the below information. a) Chemical formula C6H8O Compound is aromatic plus has two 1H NMR peaks that integrated for 3 each that are singlets (it could have more peaks in the 1H NMR b) Chemical Formula: C6H100 Compounds is conjugated 'H NMR has a signal that integrates for 6 and is a doublet IR spectra has a signal at 1730 cm-1arrow_forward
- Jaslev Propose a synthesis of the following starting from benzene and any other reagents and chemicals. No mechanisms are required. Indicate the condition for each step plus the major product for each step. More than two steps are required. Step 1 Step 2 مہد Brarrow_forwardPart C: The line formula for another branched alkane is shown below. i. In the IUPAC system what is the root or base name of this compound? ii. How many alkyl substituents are attached to the longest chain? iii. Give the IUPAC name for this compound.arrow_forwardPart D: Draw the Structural Formula for 4-ethyl-2-methylhexane Part E. Draw the Structural Formula for 1-chloro-3,3-diethylpentane (Chloro = Cl)arrow_forward
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER





