
Chemistry in Context
8th Edition
ISBN: 9780073522975
Author: American Chemical Society
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 9.11, Problem 9.30YT
Skill Building Meet DEHP
DEHP belongs to a common class of plasticizers called phthalates (THAL-ates). Phthalates are esters of phthal ic (THAL-ic) acid, an isomer of terephtha lic acid, one of the monomers used to synthesize PET.
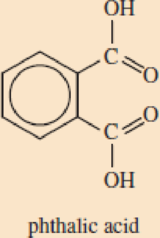
- a. Explain the meaning of the term ester.
- b. Below is the structural formula for DEHP. Circle the two ester groups in this molecule.
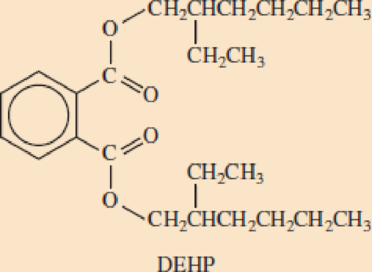
- c. Draw a structural formula for the alcohol that reacted with terephthalic acid to form this ester.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Add substituents to draw the conformer below (sighting down
the indicated bond), then rotate the back carbon to provide the
anti staggered conformer.
+
H3C
H
Ph
H
Problem 25 of 30
Drawing
Atoms, Bonds
and Rings
Charges
Tap a node to see suggestions
H
H
H
Undo
Rasat
Remove
Done
Finish update
Rotate
Submit
what temperature does a 50% (mole
fraction) of ammonia/water liquid
mixture boil at 1 atm
1) Suppose 0.1 kg ice at 0°C (273K) is in 0.5kg water at 20°C (293K). What is the change in entropy of the ice as it melts at 0°?
To produce the original "water gas" mixture, carbon (in a combustible form known as coke) is reacted with steam: 131.4 kJ + H20(g) + C(s) → CO(g) + H2(g) From this information and the equations in the previous problem, calculate the enthalpy for the combustion or carbon to form carbon dioxide.
kindly show me how to solve both parts of the same long problem. Thanks
Chapter 9 Solutions
Chemistry in Context
Ch. 9.1 - Scientific Practices Tennis Anyone? Examine this...Ch. 9.1 - Prob. 9.2CTCh. 9.4 - Prob. 9.4YTCh. 9.4 - Prob. 9.5CTCh. 9.4 - Prob. 9.6CTCh. 9.4 - Prob. 9.7CTCh. 9.4 - Prob. 9.8CTCh. 9.5 - Prob. 9.9CTCh. 9.5 - Prob. 9.11CTCh. 9.5 - Skill Building Polystyrene Possibilities Show the...
Ch. 9.6 - Skill Building Esters and Polyesters You have seen...Ch. 9.6 - Prob. 9.14CTCh. 9.7 - Skill Building Kevlar Kevlar is a polyamide used...Ch. 9.8 - Prob. 9.18CTCh. 9.8 - Your Turn 9.23 Scientific Practices Landfill...Ch. 9.9 - Prob. 9.23YTCh. 9.9 - Prob. 9.24CTCh. 9.11 - Your Turn 9.31 Scientific Practices Glass or...Ch. 9.11 - Prob. 9.29CTCh. 9.11 - Skill Building Meet DEHP DEHP belongs to a common...Ch. 9 - Prob. 1QCh. 9 - Prob. 2QCh. 9 - Prob. 3QCh. 9 - Prob. 4QCh. 9 - Prob. 5QCh. 9 - Prob. 6QCh. 9 - Prob. 7QCh. 9 - Prob. 8QCh. 9 - Prob. 9QCh. 9 - Prob. 10QCh. 9 - Prob. 11QCh. 9 - Prob. 12QCh. 9 - Prob. 13QCh. 9 - Prob. 14QCh. 9 - Prob. 15QCh. 9 - Prob. 16QCh. 9 - Prob. 17QCh. 9 - Prob. 18QCh. 9 - Prob. 19QCh. 9 - Prob. 20QCh. 9 - Prob. 21QCh. 9 - Prob. 22QCh. 9 - Prob. 23QCh. 9 - Prob. 24QCh. 9 - Prob. 25QCh. 9 - Prob. 26QCh. 9 - Prob. 27QCh. 9 - Prob. 28QCh. 9 - Prob. 29QCh. 9 - Prob. 30QCh. 9 - Prob. 31QCh. 9 - Prob. 32QCh. 9 - Prob. 33QCh. 9 - Prob. 34QCh. 9 - Prob. 35QCh. 9 - Prob. 36QCh. 9 - Prob. 37QCh. 9 - Prob. 38QCh. 9 - Prob. 39QCh. 9 - Prob. 40QCh. 9 - Prob. 41QCh. 9 - Prob. 42QCh. 9 - Prob. 43QCh. 9 - Prob. 44QCh. 9 - Prob. 45QCh. 9 - Prob. 46QCh. 9 - Prob. 47QCh. 9 - Prob. 48QCh. 9 - Prob. 49QCh. 9 - Prob. 50QCh. 9 - Prob. 51QCh. 9 - Prob. 52QCh. 9 - Prob. 53QCh. 9 - Prob. 54QCh. 9 - Prob. 55QCh. 9 - Prob. 56QCh. 9 - Prob. 57QCh. 9 - Prob. 58QCh. 9 - Prob. 59Q
Additional Science Textbook Solutions
Find more solutions based on key concepts
Identify each of the following reproductive barriers as prezygotic or postzygotic. a. One lilac species lives o...
Campbell Essential Biology with Physiology (5th Edition)
The validity of a scientific law.
Physical Universe
11. In the early 1800s, French naturalist Jean Baptiste Lamarck suggested that the best explanation for the rel...
Campbell Biology: Concepts & Connections (9th Edition)
To test your knowledge, discuss the following topics with a study partner or in writing ideally from memory. Th...
HUMAN ANATOMY
Label each statement about the polynucleotide ATGGCG as true or false. The polynucleotide has six nucleotides. ...
General, Organic, and Biological Chemistry - 4th edition
2. Define equilibrium population. Outline the conditions that must be met for a population to stay in genetic e...
Biology: Life on Earth (11th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- we were assigned to dilute 900ppm in to 18ppm by using only 250ml vol flask. firstly we did calc and convert 900ppm to 0.9 ppm to dilute in 1 liter. to begin the experiment we took 0,225g of kmno4 and dissolved in to 250 vol flask. then further we took 10 ml sample sol and dissolved in to 100 ml vol flask and put it in to a spectrometer and got value of 0.145A . upon further calc we got v2 as 50ml . need to find DF, % error (expval and accptVal), molarity, molality. please write the whole report. thank you The format, tables, introduction, procedure and observation, result, calculations, discussion and conclusionarrow_forwardQ5. Predict the organic product(s) for the following transformations. If no reaction will take place (or the reaction is not synthetically useful), write "N.R.". Determine what type of transition state is present for each reaction (think Hammond Postulate). I Br₂ CH3 F2, light CH3 Heat CH3 F₂ Heat Br2, light 12, light CH3 Cl2, light Noarrow_forwardNonearrow_forward
- In the phase diagram of steel (two components Fe and C), region A is the gamma austenite solid and region B contains the gamma solid and liquid. Indicate the degrees of freedom that the fields A and B have,arrow_forwardFor a condensed binary system in equilibrium at constant pressure, indicate the maximum number of phases that can exist.arrow_forwardPart V. Label ad match the carbons in compounds Jane and Diane w/ the corresponding peak no. in the Spectra (Note: use the given peak no. To label the carbons, other peak no are intentionally omitted) 7 4 2 -0.13 -0.12 -0.11 -0.10 -0.08 8 CI Jane 1 -0.09 5 210 200 190 180 170 160 150 140 130 120 110 100 -8 90 f1 (ppm) 11 8 172.4 172.0 f1 (ppr HO CI NH Diane 7 3 11 80 80 -80 -R 70 60 60 2 5 -8 50 40 8. 170 160 150 140 130 120 110 100 90 -0 80 70 20 f1 (ppm) 15 30 -20 20 -60 60 -0.07 -0.06 -0.05 -0.04 -0.03 -0.02 -0.01 -0.00 -0.01 10 -0.17 16 15 56 16 -0.16 -0.15 -0.14 -0.13 -0.12 -0.11 -0.10 -0.09 -0.08 -0.07 -0.06 -0.05 -0.04 17.8 17.6 17.4 17.2 17.0 f1 (ppm) -0.03 -0.02 550 106 40 30 20 20 -0.01 -0.00 F-0.01 10 0arrow_forward
- n Feb 3 A T + 4. (2 pts) Draw the structure of the major component of the Limonene isolated. Explain how you confirmed the structure. 5. (2 pts) Draw the fragment corresponding to the base peak in the Mass spectrum of Limonene. 6. (1 pts) Predict the 1H NMR spectral data of R-Limonene. Proton NMR: 5.3 pon multiplet (H Ringarrow_forwardPart VI. Ca H 10 O is the molecular formula of compound Tom and gives the in the table below. Give a possible structure for compound Tom. 13C Signals summarized C1 C2 C3 C4 C5 C6 C7 13C shift (ppm) 23.5 27.0 33.0 35.8 127 162 205 DEPT-90 + DEPT-135 + +arrow_forward2. Using the following data to calculate the value of AvapH o of water at 298K. AvapH o of water at 373K is 40.7 kJ/mol; molar heat capacity of liquid water at constant pressure is 75.2J mol-1 K-1 and molar heat capacity of water vapor at constant pressure is 33.6 J mol-1 K-1.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Quantum Mechanics - Part 1: Crash Course Physics #43; Author: CrashCourse;https://www.youtube.com/watch?v=7kb1VT0J3DE;License: Standard YouTube License, CC-BY