
Concept explainers
(a)
Interpretation:
Concept introduction:
The heat of the reaction
The formula to calculate
Or,
The bond energy of reactants is positive and the bond energy of products is negative.
The standard enthalpy of reaction is calculated by the summation of standard enthalpy of formation of the product minus the summation of standard enthalpy of formation of product at the standard conditions. The formula to calculate the standard enthalpy of reaction
Here, m and n are the stoichiometric coefficients of reactants and product in the balanced chemical equation.
(a)
Answer to Problem 9.91P
Explanation of Solution
The given chemical equation for the gas-phase hydration of ethylene to ethanol is as follows:
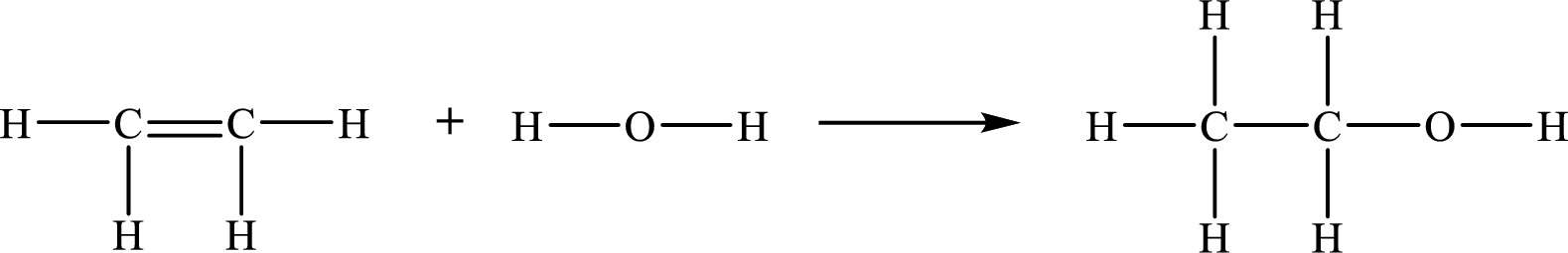
The number of broken bonds is
The number of bonds formed is
The formula to the enthalpy of the given reaction is as follows:
Substitute
The formula to calculate the standard enthalpy of reaction
Substitute
(b)
Interpretation:
Concept introduction:
The heat of the reaction
The formula to calculate
Or,
The bond energy of reactants is positive and the bond energy of products is negative.
(b)
Answer to Problem 9.91P
Explanation of Solution
The given chemical equation for the hydrolysis of ethylene glycol is as follows:
The number of broken bonds is
The number of bonds formed is
The formula to the enthalpy of the given reaction is as follows:
Substitute
(c)
Interpretation:
The reason for the relatively closer value for the hydration in part (a) but not that close for the hydrolysis in part (b) is to be determined.
Concept introduction:
Bond energy is the amount of energy needed to break a bond between two gaseous atoms. Bond energy is measured in
The formula to calculate
Or,
The bond energy of reactants is positive and the bond energy of products is negative.
(c)
Answer to Problem 9.91P
In part (b), the same number of similar bonds are broken in the reactant side and formed in the product side so the value of the enthalpy change is zero. Bond energy is generally specific for a bond and remains the same in different molecules.
Explanation of Solution
Bond energy is generally specific for a bond and depends upon the strength of the bond. The bond energy value is the average value so remain same in different molecules.
In part (b), the same number of similar bonds are broken in the reactant side and formed in the product side so the value of the enthalpy change is zero.
For example, the value of bond energy of
The bond energy value is the average value so the bond energy of the same bond in different molecules remains same.
Want to see more full solutions like this?
Chapter 9 Solutions
CHEMISTRY >CUSTOM<
- Predict the organic product of Y that is formed in the reaction below, and draw the skeletal ("line") structures of the missing organic product. Please include all steps & drawings & explanations.arrow_forwardPlease choose the best reagents to complete the following reactionarrow_forwardProblem 6-17 Look at the following energy diagram: Energy Reaction progress (a) Is AG for the reaction positive or negative? Label it on the diagram. (b) How many steps are involved in the reaction? (c) How many transition states are there? Label them on the diagram. Problem 6-19 What is the difference between a transition state and an intermediate? Problem 6-21 Draw an energy diagram for a two-step reaction with Keq > 1. Label the overall AG°, transition states, and intermediate. Is AG° positive or negative? Problem 6-23 Draw an energy diagram for a reaction with Keq = 1. What is the value of AG° in this reaction?arrow_forward
- Problem 6-37 Draw the different monochlorinated constitutional isomers you would obtain by the radical chlorination of the following compounds. (b) (c) Problem 6-39 Show the structure of the carbocation that would result when each of the following alkenes reacts with an acid, H+. (a) (b) (c)arrow_forwardPlease draw the major product of this reaction. Ignore inorganic byproducts and the carboxylic side productarrow_forwardPlease draw the major product of this reaction.arrow_forward
- Draw the major product of this reaction.arrow_forwardidentify the carbonyl compound that is incapable of forming an enolate ionarrow_forwardpredict the product formed by the reaction of one mole each of cyclohex-2-en-1-one and lithium diethylcuprate. Assume a hydrolysis step follows the additionarrow_forward
- Please handwriting for questions 1 and 3arrow_forwardIs (CH3)3NHBr an acidic or basic salt? What happens when dissolved in aqueous solution? Doesn't it lose a Br-? Does it interact with the water? Please advise.arrow_forward© Macmilla Finish resonance structure 3 Select Draw Templates More C H N 0 H H S Erase Which structure is the most stable (lowest energy) resonance contributor? The structure with the positive charge on nitrogen and negative charges on oxygen and sulfur. All structures are equal in stability. The structure with the positive charge on nitrogen and negative charges on sulfur and carbon. The structure with the positive charge on nitrogen and negative charges on oxygen and carbon. Q2Qarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





