
Concept explainers
(a)
Interpretation:
For the given reaction, the major product is to be determined. The complete, detailed mechanism is to be drawn.
Concept introduction:
When the attacking species is an alcohol which is a weak base and weak nucleophile, it favors
Answer to Problem 9.81P
The complete, detailed mechanism and the major product for the given reaction is shown below:
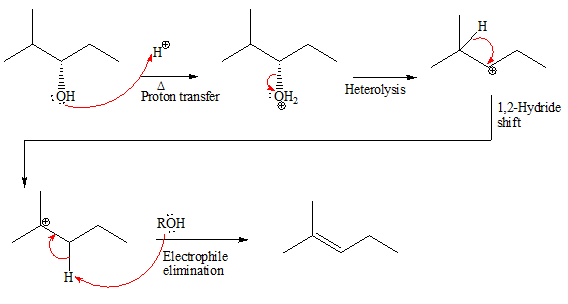
Explanation of Solution
The given reacting species are shown below:
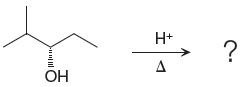
In the given reaction, attacking species is an alcohol which is a weak nucleophile. Also, the leaving group can be water under acidic conditions which is an excellent leaving group. Also, the solvent is an alcohol. Therefore, the given reaction favors

The complete, detailed mechanism and the major product for the given reaction is drawn on the basis of reacting species.
(b)
Interpretation:
For the given reaction, the major product is to be determined. The complete, detailed mechanism is to be drawn.
Concept introduction:
When attacking species is an alcohol which is a weak base and weak nucleophile, it favors
Answer to Problem 9.81P
The complete, detailed mechanism and the major product for the given reaction is shown below:
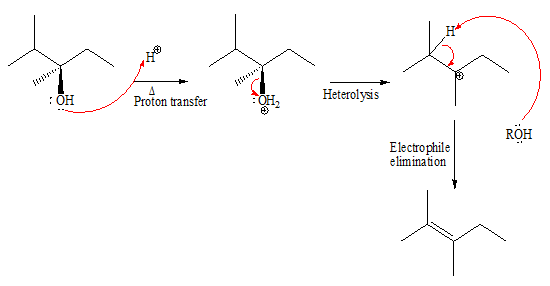
Explanation of Solution
The given reacting species are shown below,
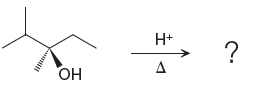
In the given reaction, attacking species is an alcohol which is a weak nucleophile. Also, the leaving group can be water under acidic conditions which is an excellent leaving group. Also, the solvent is an alcohol. Therefore, the given reaction favors

The complete, detailed mechanism and the major product for the given reaction are drawn on the basis of reacting species.
(c)
Interpretation:
For the given reaction, the major product is to be determined. The complete, detailed mechanism is to be drawn.
Concept introduction:
When attacking species is an alcohol which is a weak base and weak nucleophile, it favors
Answer to Problem 9.81P
The complete, detailed mechanism and the major product for the given reaction is shown below:
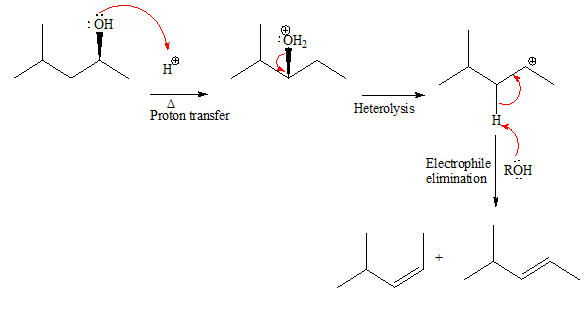
Explanation of Solution
The given reacting species are shown below,
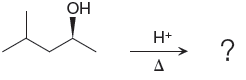
In the given reaction, attacking species is an alcohol which is a weak nucleophile. Also, the leaving group can be water under acidic conditions which is an excellent leaving group. Also, the solvent is an alcohol. Therefore, the given reaction favors 
The complete, detailed mechanism and the major product for the given reaction are drawn on the basis of reacting species.
(d)
Interpretation:
The major product of the given reaction is to be determined. The complete, detailed mechanism is to be drawn.
Concept introduction:
When attacking species is an alcohol which is a weak base and weak nucleophile, it favors
Answer to Problem 9.81P
The complete, detailed mechanism and the major product for the given reaction is shown below,
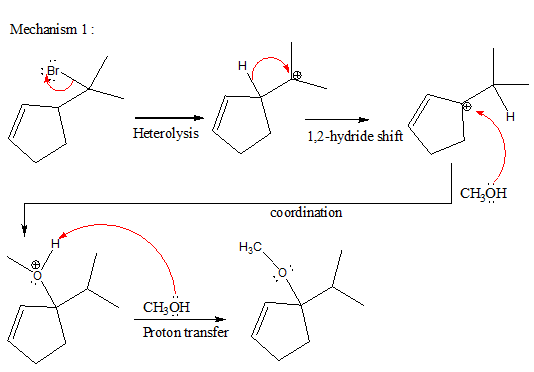
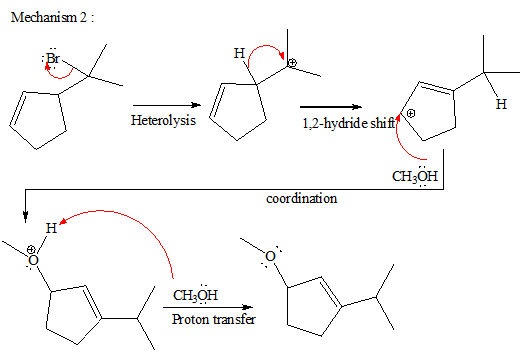
Explanation of Solution
The given reacting species are shown below,
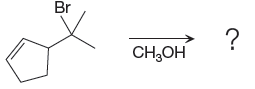
In the given reaction, attacking species is an alcohol which is a weak nucleophile. Also, the leaving group can be water under acidic conditions which is an excellent leaving group. Also, the solvent is an alcohol. Therefore, the given reaction favors


The complete, detailed mechanism and the major product for given reaction are drawn on the basis of reacting species.
(e)
Interpretation:
For the given reaction, the major product is to be determined. The complete, detailed mechanism is to be drawn.
Concept introduction:
The strong nucleophile and weak base favors
Answer to Problem 9.81P
The complete, detailed mechanism and product of the given reaction is as shown below:
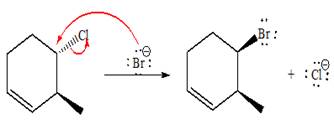
Explanation of Solution
The given reacting species are shown below,
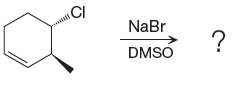
The Br- is a strong nucleophile but a weak base that favors
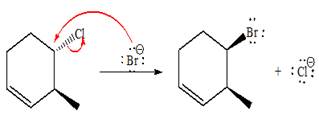
The complete, detailed mechanism and the major product for the given reaction are drawn on the basis of reacting species.
(f)
Interpretation:
For the given reaction, the major product is to be determined. The complete, detailed mechanism is to be drawn.
Concept introduction:
If the attacking species is a strong bulky base but has low concentration it favors
Answer to Problem 9.81P
The complete, detailed mechanism and the products of the given reaction are shown below,
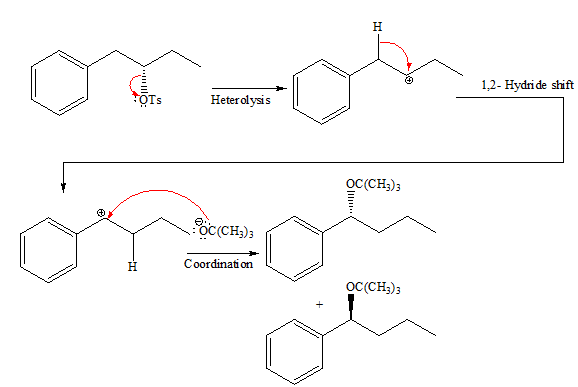
Explanation of Solution
The given reacting species are shown below,
In the given reaction, the attacking species is a strong bulky base so it favors

The complete, detailed mechanism and the major product for the given reaction is drawn on the basis of reacting species.
(g)
Interpretation:
For the given reaction, the major product is to be determined. The complete, detailed mechanism is to be drawn.
Concept introduction:
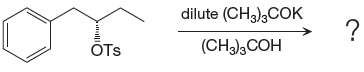
When attacking species is an alcohol which is a weak base and weak nucleophile, it favors
Answer to Problem 9.81P
The complete, detailed mechanism and the major product for the given reaction is shown below,
Explanation of Solution
The given reacting species are shown below:
In the given reaction, attacking species is a weak nucleophile and weak base. Also, the leaving group

The complete, detailed mechanism and the major product for the given reaction are drawn on the basis of reacting species.
Want to see more full solutions like this?
Chapter 9 Solutions
Organic Chemistry: Principles and Mechanisms (Second Edition)
- What are the angles a and b in the actual molecule of which this is a Lewis structure? H H C H- a -H b H Note for advanced students: give the ideal angles, and don't worry about small differences from the ideal groups may have slightly different sizes. a = b = 0 °arrow_forwardWhat are the angles a and b in the actual molecule of which this is a Lewis structure? :0: HCOH a Note for advanced students: give the ideal angles, and don't worry about small differences from the ideal that might be caused by the fact that different electron groups may have slightly different sizes. a = 0 b=0° Sarrow_forwardDetermine the structures of the missing organic molecules in the following reaction: + H₂O +H OH O OH +H OH X Note: Molecules that share the same letter have the exact same structure. In the drawing area below, draw the skeletal ("line") structure of the missing organic molecule X. Click and drag to start drawing a structure.arrow_forward
- Identify the missing organic reactant in the following reaction: x + x O OH H* + ☑- X H+ O O Х Note: This chemical equation only focuses on the important organic molecules in the reaction. Additional inorganic or small-molecule reactants or products (like H₂O) are not shown. In the drawing area below, draw the skeletal ("line") structure of the missing organic reactant X. Click and drag to start drawing a structure. Carrow_forwardCH3O OH OH O hemiacetal O acetal O neither O 0 O hemiacetal acetal neither OH hemiacetal O acetal O neither CH2 O-CH2-CH3 CH3-C-OH O hemiacetal O acetal CH3-CH2-CH2-0-c-O-CH2-CH2-CH3 O neither HO-CH2 ? 000 Ar Barrow_forwardWhat would be the best choices for the missing reagents 1 and 3 in this synthesis? 1. PPh3 2 2. n-BuLi 3 Draw the missing reagents in the drawing area below. You can draw them in any arrangement you like. • Do not draw the missing reagent 2. If you draw 1 correctly, we'll know what it is. • Note: if one of your reagents needs to contain a halogen, use bromine. Explanation Check Click and drag to start drawing a structure.arrow_forward
- Predict the products of this organic reaction: NaBH3CN + NH2 ? H+ Click and drag to start drawing a structure. ×arrow_forwardPredict the organic products that form in the reaction below: + OH +H H+ ➤ ☑ X - Y Note: You may assume you have an excess of either reactant if the reaction requires more than one of those molecules to form the products. In the drawing area below, draw the skeletal ("line") structures of the missing organic products X and Y. You may draw the structures in any arrangement that you like, so long as they aren't touching. Click and drag to start drawing a structure. Garrow_forwardPredict the organic products that form in the reaction below: OH H+ H+ + ☑ Y Note: You may assume you have an excess of either reactant if the reaction requires more than one of those molecules to form the products. In the drawing area below, draw the skeletal ("line") structures of the missing organic products X and Y. You may draw the structures in any arrangement that you like, so long as they aren't touching. Click and drag to start drawing a structure. ✓ marrow_forward
- Determine the structures of the missing organic molecules in the following reaction: + H₂O +H H+ Y Z ☑ ☑ Note: Molecules that share the same letter have the exact same structure. In the drawing area below, draw the skeletal ("line") structures of the missing organic molecules X, Y, and Z. You may draw the structures in any arrangement that you like, so long as they aren't touching. Molecule X shows up in multiple steps, but you only have to draw its structure once. Click and drag to start drawing a structure. AP +arrow_forwardPlease help, this is all the calculations i got!!! I will rate!!!Approx mass of KMnO in vial: 3.464 4 Moss of beaker 3×~0. z Nax200: = 29.9219 Massof weacerv after remosimgain N2C2O4. Need to fill in all the missing blanks. ง ง Approx mass of KMnO4 in vials 3.464 Mass of beaker + 3x ~0-304: 29.9219 2~0.20 Miss of beaker + 2x- 29.7239 Mass of beaker + 1x~0.2g Naz (204 29-5249 Mass of beaver after removing as qa Na₂ C₂O T1 T2 T3 Final Buiet reading Initial butet reading (int)) Hass of NaOr used for Titration -reading (mL) calculation Results: 8.5ml 17mL 27.4mL Oml Om Oml T1 T2 T3 Moles of No CO Moles of KMO used LOF KM. O used Molenty of KMNO Averagem Of KMOWLarrow_forwardDraw the skeletal ("line") structure of 2-hydroxy-4-methylpentanal. Click and drag to start drawing a structure. Xarrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
