
Concept explainers
(a)
Interpretation:
The complete, detailed mechanism for the reaction is to be provided, and the products are to be predicted including stereochemistry, where appropriate. It is be determined whether the reaction will yield exclusively one product or a mixture of products. If the reaction will yield a mixture of products, then the major product is to be determined.
Concept introduction:
In order to predict the outcome of the given reaction, four factors are considered.
First is to determine if the substrate molecule has a suitable leaving group. If the leaving group is suitable, then evaluate the type of carbon bonded to the leaving group. For an
Answer to Problem 9.63P
The complete, detailed mechanism for the reaction and the products (major) including the stereochemistry are given below:
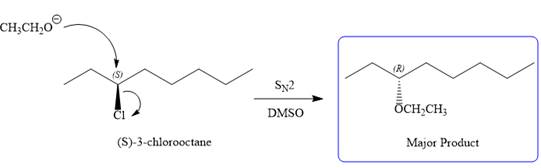
E2 pathway:
Explanation of Solution
The given reaction is
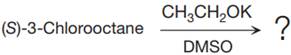
The structure of
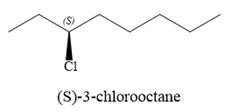
The substrate has one site for substitution or elimination as chlorine is a moderately good leaving group. The chlorine atom is attached to an
the corresponding ions:
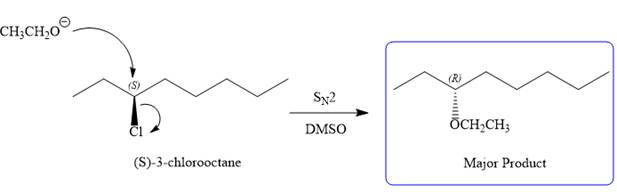
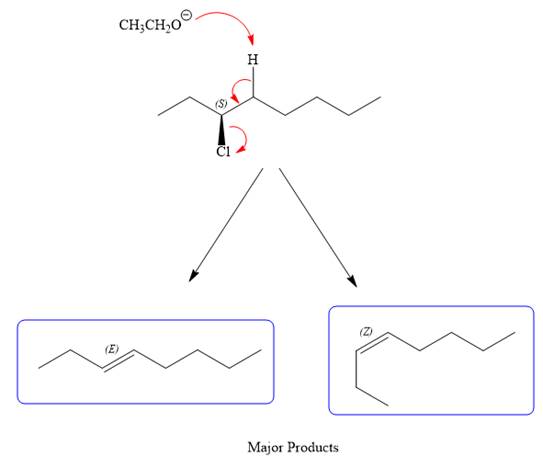
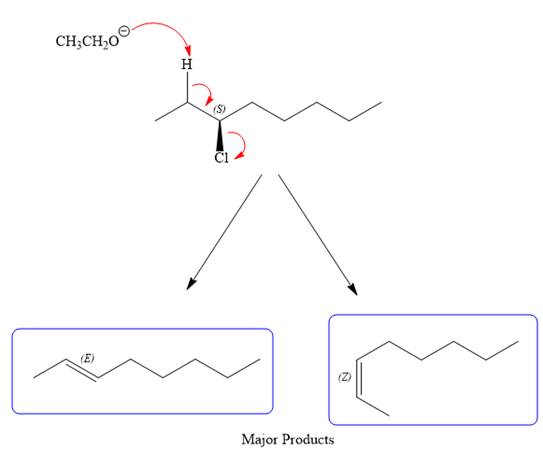
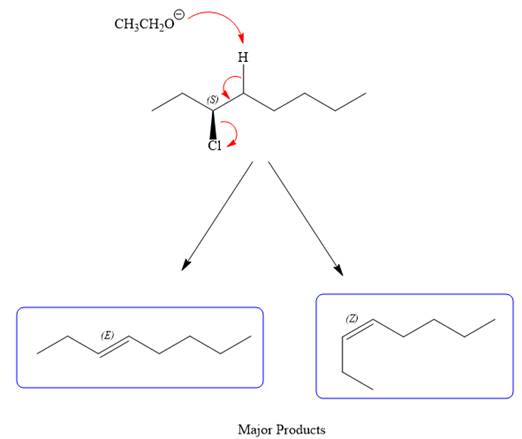
For the E2 product, the base abstracts the alpha hydrogen atom, which results in the formation of a mixture of products. The substrate has two types of alpha hydrogen atoms attached at C2 and C4 carbon atoms. Thus, in all four different products (alkenes) are possible for the
Note the stereochemistry, which is governed by the attack of the nucleophile from the side opposite the leaving group to yield major product in case of
As for the E2 reaction, as there are two different hydrogen atoms attached on either sides of the carbon atom with the leaving group, there could be four stereoisomers for the reaction. All four will be the major products.
The outcome of any given reaction can be predicted by considering the factors like nature of the leaving group, substrate, strength of the reagent used, solvent, and temperature.
(b)
Interpretation:
The complete, detailed mechanism for the reaction is to be provided and the products are to be predicted including stereochemistry where appropriate. It is be determined whether the reaction will yield exclusively one product or a mixture of products. If the reaction will yield a mixture of products, then the major product is to be determined.
Concept introduction:
In order to predict the outcome of the given reaction, four factors are considered.
First is to determine if the substrate molecule has a suitable leaving group. If the leaving group is suitable, then evaluate the type of carbon bonded to the leaving group. For an
Answer to Problem 9.63P
The complete, detailed mechanism for the reaction and the products (major) including the stereochemistry are given below:
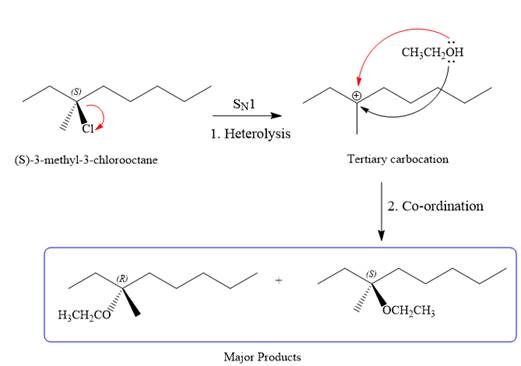
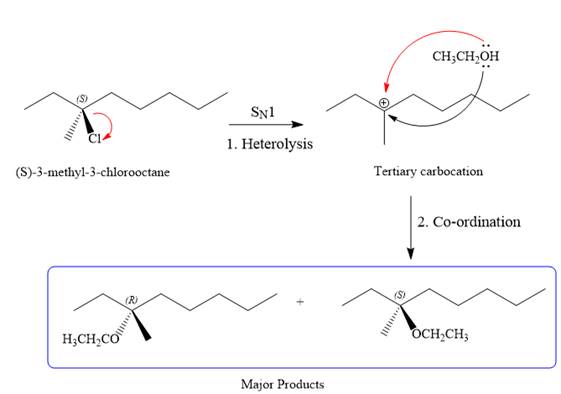
SN1 pathway:
E1 pathway:
Explanation of Solution
The given reaction is

The structure of
The substrate has one site for substitution or elimination as chlorine is a moderately good leaving group. The chlorine atom is attached to an
As the leaving group is attached to a tertiary carbon atom,
First step in the
Step two is the attack of the nucleophile on the carbocation from both the sides to generate mixture of stereoisomers if the substrate has a chiral center and the reaction occurs at the chiral center. The C3 carbon atom where the reaction occurs is a chiral carbon atom. Thus, a mixture of stereoisomers is produced as the racemic mixture; thus, two produces are formed as major products in the
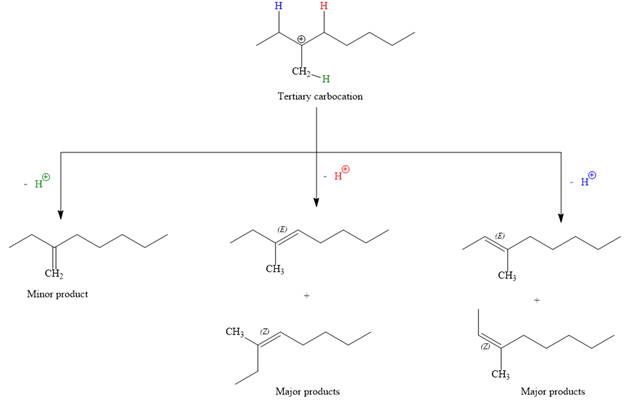
For the E1 product, the first step is common, that is, formation of a tertiary carbocation.
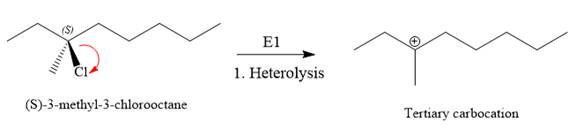
In the second step, the hydrogen atom from the adjacent carbon atom of the carbocation is abstracted, resulting into an alkene. The tertiary carbocation formed above has three different adjacent hydrogen atoms.
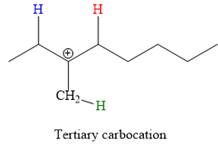
Elimination usually occurs so as to produce the most stable (the most substituted) alkene as the major product. Thus, abstraction of the green hydrogen atom by the base would result in the least substituted alkene, and therefore, it will be the minor product.
Abstraction of red and blue protons would result in four possible products, all of which are major products.
The complete, detailed mechanism for the E1 reaction is given below:
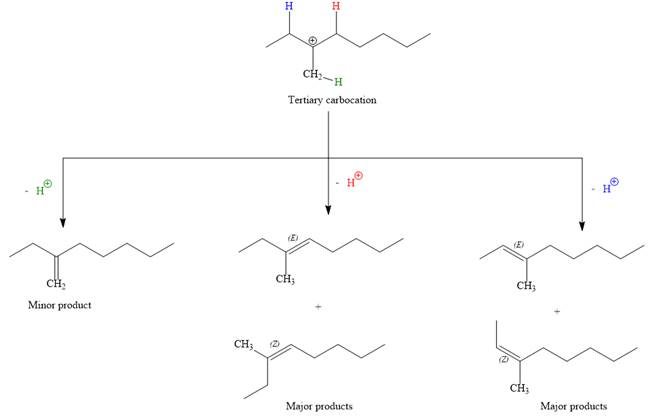
Note the stereochemistry, there are four major products (trisubstituted alkenes), and one minor product (disubstituted alkene) formed in the above mechanism.
The outcome of any given reaction can be predicted by considering the factors like nature of the leaving group, substrate, strength of the reagent used, solvent, and temperature.
(c)
Interpretation:
The complete, detailed mechanism for the reaction is to be provided and the products are to be predicted including stereochemistry where appropriate. It is be determined whether the reaction will yield exclusively one product or a mixture of products. If the reaction will yield a mixture of products, then the major product is to be determined.
Concept introduction:
In order to predict the outcome of the given reaction, four factors are considered.
First is to determine if the substrate molecule has a suitable leaving group. If the leaving group is suitable, then evaluate the type of carbon bonded to the leaving group. For an
Answer to Problem 9.63P
The complete, detailed mechanism for the reaction and the products (major) including the stereochemistry are given below:
Explanation of Solution
The given reaction is
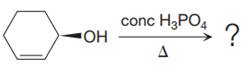
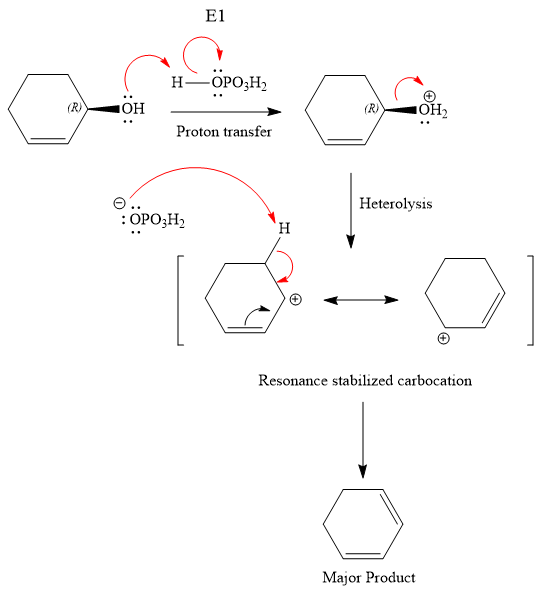
The substrate has one site for substitution or elimination; however hydroxyl group is not a good leaving group. The chlorine atom is attached to an
The reagent used is a phosphoric acid, which is a strong acid. It will protonate the oxygen atom in the hydroxyl group and convert it into a good leaving group. Elimination reactions are generally favored if the reaction conditions includes heat. This rules out both the substitution reactions. And as the hydroxyl group will be protonated by the strong acid, in the next step, it will be removed to generate a resonance stabilized secondary carbocation. Thus, the E1 reaction is highly favored. In the next step, the hydrogen atom adjacent to the carbocation is removed to form an
The complete, detailed mechanism is shown below:
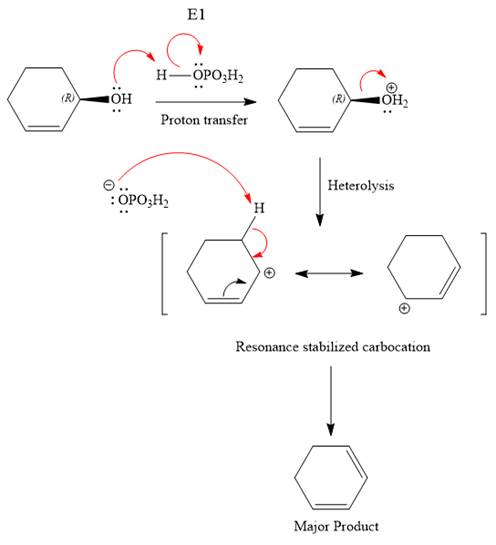
The outcome of any given reaction can be predicted by considering the factors like nature of the leaving group, substrate, strength of the reagent used, solvent, and temperature.
(d)
Interpretation:
The complete, detailed mechanism for the reaction is to be provided and the products are to be predicted including stereochemistry where appropriate. It is be determined whether the reaction will yield exclusively one product or a mixture of products. If the reaction will yield a mixture of products, then the major product is to be determined.
Concept introduction:
In order to predict the outcome of the given reaction, four factors are considered.
First is to determine if the substrate molecule has a suitable leaving group. If the leaving group is suitable, then evaluate the type of carbon bonded to the leaving group. For an
Answer to Problem 9.63P
The complete, detailed mechanism for the reaction and the products (major) including the stereochemistry are given below:
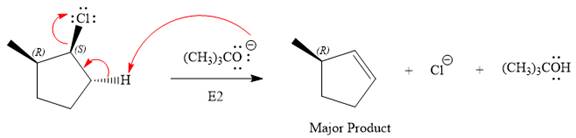
Explanation of Solution
The given reaction is
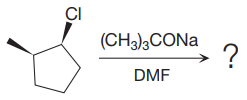
The substrate has one site for substitution or elimination as chlorine is a moderately good leaving group. The chlorine atom is attached to an
the corresponding ions:
Here,
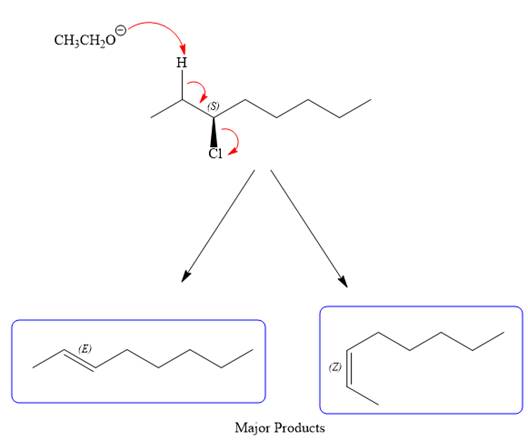
For the E2 product, the base abstracts the alpha hydrogen atom which would result in the formation of a mixture of products. But since the base is a bulky base, the proton, which is least sterically hindered, will be abstracted to form the major product.
The complete, detailed mechanisms is shown below:

The outcome of any given reaction can be predicted by considering the factors like nature of the leaving group, substrate, strength of the reagent used, solvent, and temperature.
(e)
Interpretation:
The complete, detailed mechanism for the reaction is to be provided and the products are to be predicted including stereochemistry where appropriate. It is be determined whether the reaction will yield exclusively one product or a mixture of products. If the reaction will yield a mixture of products, then the major product is to be determined.
Concept introduction:
In order to predict the outcome of the given reaction, four factors are considered.
First is to determine if the substrate molecule has a suitable leaving group. If the leaving group is suitable, then evaluate the type of carbon bonded to the leaving group. For an
Answer to Problem 9.63P
The complete, detailed mechanism for the reaction and the products (major) including the stereochemistry are given below:
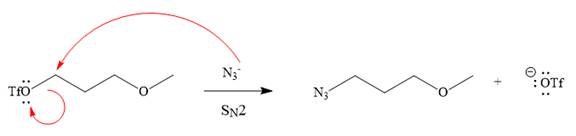
Explanation of Solution
The given reaction is
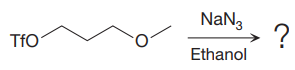
The substrate has one site for substitution or elimination as the
The
The complete, detailed mechanism is shown below:

As the reaction yields a single product, it is the major product.
The outcome of any given reaction can be predicted by considering the factors like nature of the leaving group, substrate, strength of the reagent used, solvent, and temperature.
(f)
Interpretation:
The complete, detailed mechanism for the reaction is to be provided and the products are to be predicted including stereochemistry where appropriate. It is be determined whether the reaction will yield exclusively one product or a mixture of products. If the reaction will yield a mixture of products, then the major product is to be determined.
Concept introduction:
In order to predict the outcome of the given reaction, four factors are considered.
First is to determine if the substrate molecule has a suitable leaving group. If the leaving group is suitable, then evaluate the type of carbon bonded to the leaving group. For an
Answer to Problem 9.63P
The complete, detailed mechanism for the reaction and the products (major) including the stereochemistry are given below:
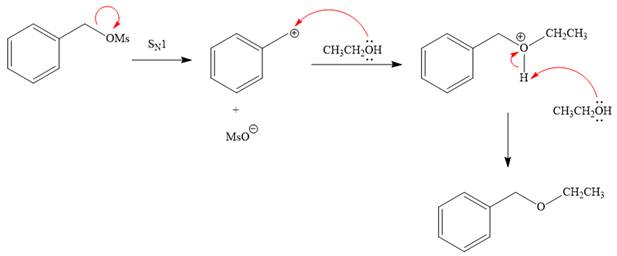
Explanation of Solution
The given reaction is
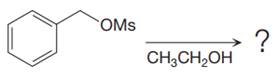
The substrate has one site for substitution or elimination, which is the
The attacking species and the solvent for the reaction is the same, that is, ethanol, which serves as a weak nucleophile and a polar protic solvent. This strongly favors
The first step in the
The complete, detailed mechanism is shown below:
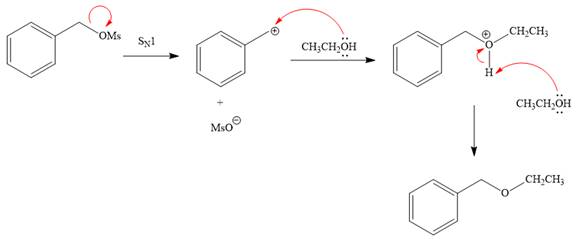
The outcome of any given reaction can be predicted by considering the factors like nature of the leaving group, substrate, strength of the reagent used, solvent, and temperature.
Want to see more full solutions like this?
Chapter 9 Solutions
ORGANIC CHEMISTRY PRINCIPLES & MECHANISM
- At the end of the silica gel production process, color changes occur during drying. Explain these color changes.arrow_forwardIf CoCl2/H2O is dissolved in a mixture of H2O and concentrated HCl in a test tube, the tube is gently heated over a flame to approximately 80°C and then cooled externally. Explain the color changes that occur.arrow_forwardWhen producing silica gel, color changes occur at the end of the drying process. Explain these color changes.arrow_forward
- Design experiments in UV-Vis to figure the optimal mole ratio of copper (1:1, 2:1, 3:1 and etc)versus ethambutol using all necessary chemicals including dihydrochloride and copper nitrate hemipentahydrate and sodium hydroxide. Show how UV-Vis absorbance and maximum wavelength would change in responsearrow_forwardCorrect each molecule in the drawing area below so that it has the condensed structure it would have if it were dissolv a 0.1 M aqueous solution of HCI. If there are no changes to be made, check the No changes box under the drawing area. No changes. HO—CH,—C—CH,—OH X 5 2 2 2 HO–CH,—CH,—C—CH,—OH Explanation Check Center Accessi ©2025 on 5 Carrow_forwardMake the calculations to prepare 2M H2SO4, from concentrated H2SO4 (98%; density: 1.84 g/mL).arrow_forward
- H CH3 CH3 b) Write the products of your compound and the following reagents. If the reaction would not work for your compound, write "no reaction" and explain the problem. NaCN H* H₂NNHCH5 H* -à NaBH -à CH2MgBr Cro₁₂ --à H3O+ -à c) Would your compound give a positive Tollen's test? Why or why not?arrow_forwardHomework 4 Chem 204 Dr. Hellwig Consider this compound, which will be referred to as "your compound". a) Name your compound according to the IUPAC system. Include stereochemistry (E/Z/R/S) H CH3 CH3arrow_forwardWhat is the mechanism for this?arrow_forward
- 21.50 Determine the combinations of haloalkane(s) and alkoxide(s) that could be used to synthesize the following ethers through Williamson ether synthesis. (a) (c) (d) (e) (f) H₂COarrow_forward1. Arrange the following in order of increasing bond energy (lowest bond energy first, highest bond energy last). Provide your rationale. C=C, C-F, C=C, C-N, C-C List the bond order for each example.arrow_forwardWhat is the major enolate formed when treated with LDA? And why that one?arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

