
Concept explainers
(a)
Interpretation:
The complete, detailed mechanism for the reaction is to be provided, and the products are to be predicted including stereochemistry, where appropriate. It is be determined whether the reaction will yield exclusively one product or a mixture of products. If the reaction will yield a mixture of products, then the major product is to be determined.
Concept introduction:
In order to predict the outcome of the given reaction, four factors are considered.
First is to determine if the substrate molecule has a suitable leaving group. If the leaving group is suitable, then evaluate the type of carbon bonded to the leaving group. For an
Answer to Problem 9.63P
The complete, detailed mechanism for the reaction and the products (major) including the stereochemistry are given below:
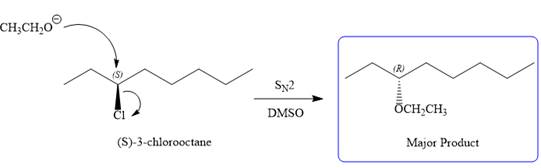
E2 pathway:
Explanation of Solution
The given reaction is
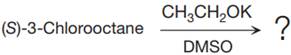
The structure of
The substrate has one site for substitution or elimination as chlorine is a moderately good leaving group. The chlorine atom is attached to an
the corresponding ions:
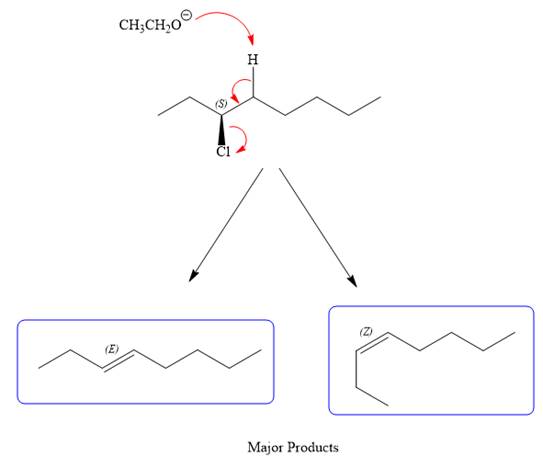
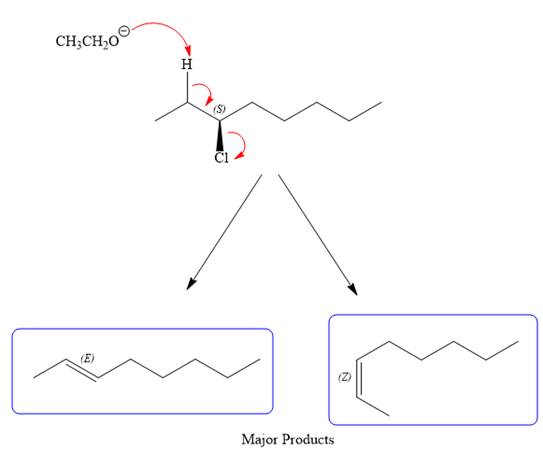
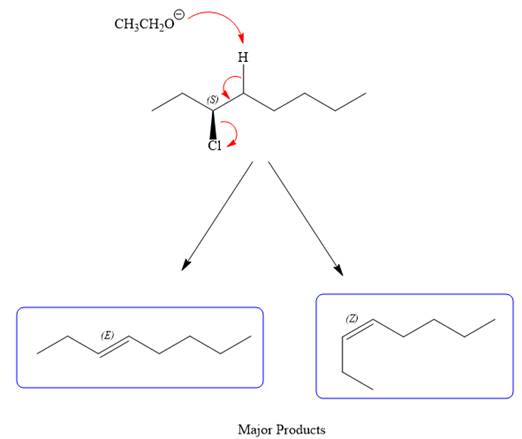
For the E2 product, the base abstracts the alpha hydrogen atom, which results in the formation of a mixture of products. The substrate has two types of alpha hydrogen atoms attached at C2 and C4 carbon atoms. Thus, in all four different products (alkenes) are possible for the
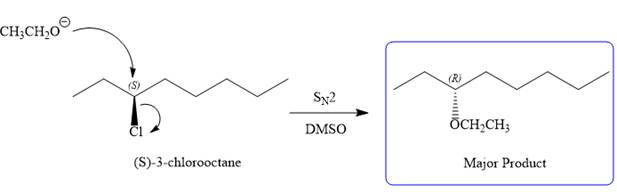
Note the stereochemistry, which is governed by the attack of the nucleophile from the side opposite the leaving group to yield major product in case of
As for the E2 reaction, as there are two different hydrogen atoms attached on either sides of the carbon atom with the leaving group, there could be four stereoisomers for the reaction. All four will be the major products.
The outcome of any given reaction can be predicted by considering the factors like nature of the leaving group, substrate, strength of the reagent used, solvent, and temperature.
(b)
Interpretation:
The complete, detailed mechanism for the reaction is to be provided and the products are to be predicted including stereochemistry where appropriate. It is be determined whether the reaction will yield exclusively one product or a mixture of products. If the reaction will yield a mixture of products, then the major product is to be determined.
Concept introduction:
In order to predict the outcome of the given reaction, four factors are considered.
First is to determine if the substrate molecule has a suitable leaving group. If the leaving group is suitable, then evaluate the type of carbon bonded to the leaving group. For an
Answer to Problem 9.63P
The complete, detailed mechanism for the reaction and the products (major) including the stereochemistry are given below:
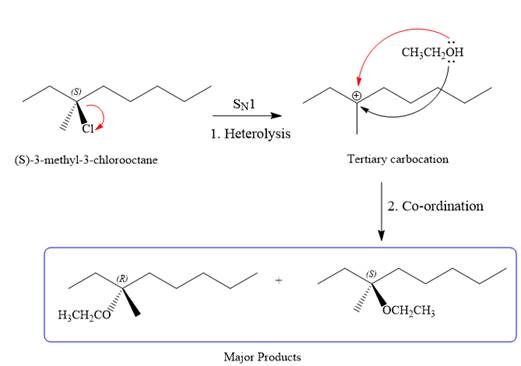
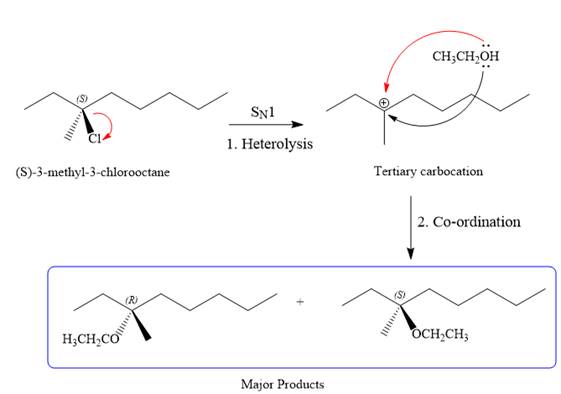
SN1 pathway:
E1 pathway:
Explanation of Solution
The given reaction is

The structure of
The substrate has one site for substitution or elimination as chlorine is a moderately good leaving group. The chlorine atom is attached to an
As the leaving group is attached to a tertiary carbon atom,
First step in the
Step two is the attack of the nucleophile on the carbocation from both the sides to generate mixture of stereoisomers if the substrate has a chiral center and the reaction occurs at the chiral center. The C3 carbon atom where the reaction occurs is a chiral carbon atom. Thus, a mixture of stereoisomers is produced as the racemic mixture; thus, two produces are formed as major products in the
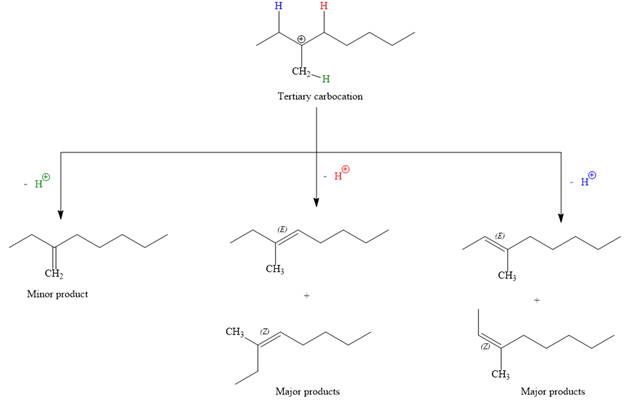
For the E1 product, the first step is common, that is, formation of a tertiary carbocation.
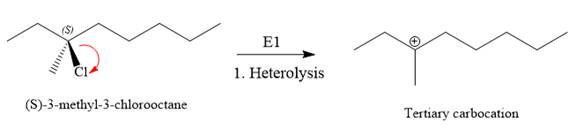
In the second step, the hydrogen atom from the adjacent carbon atom of the carbocation is abstracted, resulting into an alkene. The tertiary carbocation formed above has three different adjacent hydrogen atoms.
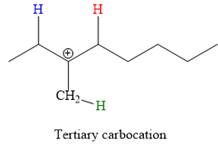
Elimination usually occurs so as to produce the most stable (the most substituted) alkene as the major product. Thus, abstraction of the green hydrogen atom by the base would result in the least substituted alkene, and therefore, it will be the minor product.
Abstraction of red and blue protons would result in four possible products, all of which are major products.
The complete, detailed mechanism for the E1 reaction is given below:
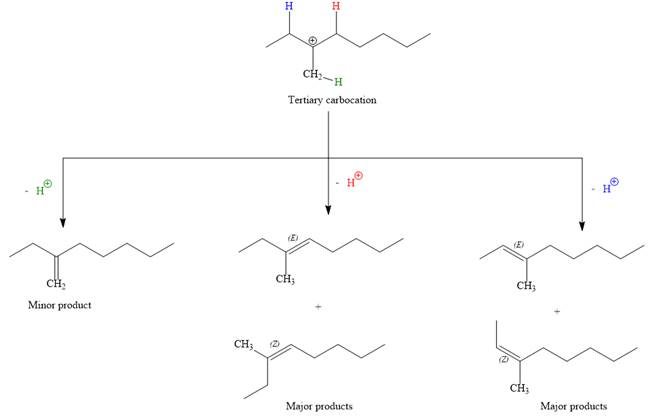
Note the stereochemistry, there are four major products (trisubstituted alkenes), and one minor product (disubstituted alkene) formed in the above mechanism.
The outcome of any given reaction can be predicted by considering the factors like nature of the leaving group, substrate, strength of the reagent used, solvent, and temperature.
(c)
Interpretation:
The complete, detailed mechanism for the reaction is to be provided and the products are to be predicted including stereochemistry where appropriate. It is be determined whether the reaction will yield exclusively one product or a mixture of products. If the reaction will yield a mixture of products, then the major product is to be determined.
Concept introduction:
In order to predict the outcome of the given reaction, four factors are considered.
First is to determine if the substrate molecule has a suitable leaving group. If the leaving group is suitable, then evaluate the type of carbon bonded to the leaving group. For an
Answer to Problem 9.63P
The complete, detailed mechanism for the reaction and the products (major) including the stereochemistry are given below:
Explanation of Solution
The given reaction is
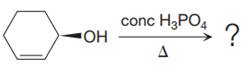
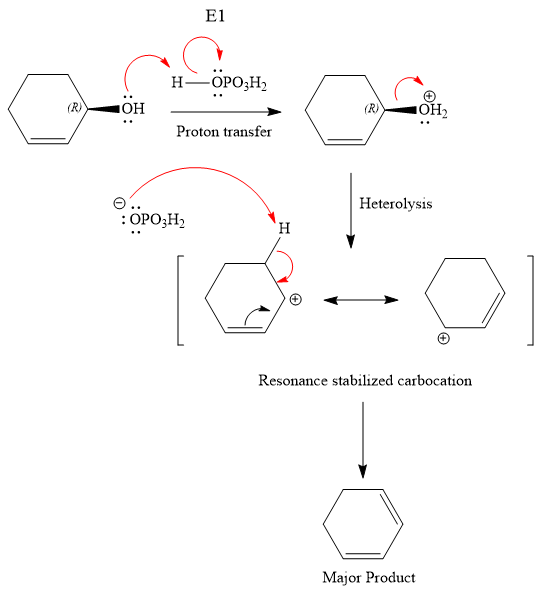
The substrate has one site for substitution or elimination; however hydroxyl group is not a good leaving group. The chlorine atom is attached to an
The reagent used is a phosphoric acid, which is a strong acid. It will protonate the oxygen atom in the hydroxyl group and convert it into a good leaving group. Elimination reactions are generally favored if the reaction conditions includes heat. This rules out both the substitution reactions. And as the hydroxyl group will be protonated by the strong acid, in the next step, it will be removed to generate a resonance stabilized secondary carbocation. Thus, the E1 reaction is highly favored. In the next step, the hydrogen atom adjacent to the carbocation is removed to form an
The complete, detailed mechanism is shown below:
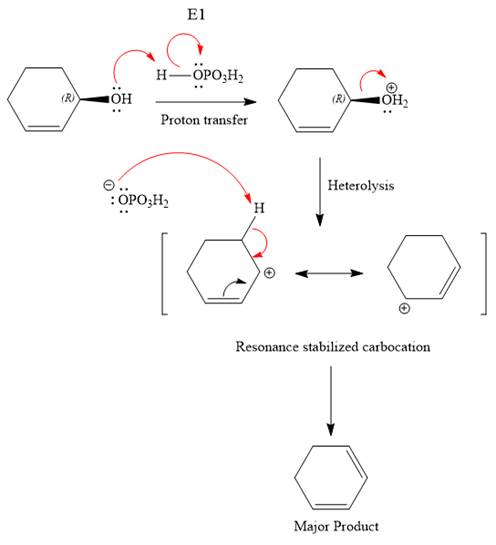
The outcome of any given reaction can be predicted by considering the factors like nature of the leaving group, substrate, strength of the reagent used, solvent, and temperature.
(d)
Interpretation:
The complete, detailed mechanism for the reaction is to be provided and the products are to be predicted including stereochemistry where appropriate. It is be determined whether the reaction will yield exclusively one product or a mixture of products. If the reaction will yield a mixture of products, then the major product is to be determined.
Concept introduction:
In order to predict the outcome of the given reaction, four factors are considered.
First is to determine if the substrate molecule has a suitable leaving group. If the leaving group is suitable, then evaluate the type of carbon bonded to the leaving group. For an
Answer to Problem 9.63P
The complete, detailed mechanism for the reaction and the products (major) including the stereochemistry are given below:
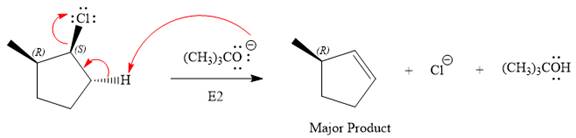
Explanation of Solution
The given reaction is
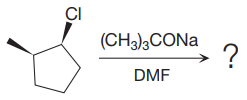
The substrate has one site for substitution or elimination as chlorine is a moderately good leaving group. The chlorine atom is attached to an
the corresponding ions:
Here,
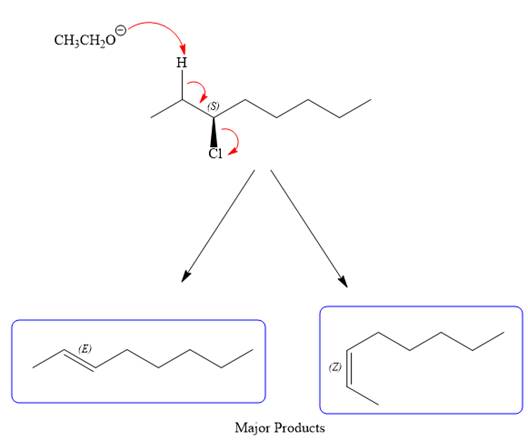
For the E2 product, the base abstracts the alpha hydrogen atom which would result in the formation of a mixture of products. But since the base is a bulky base, the proton, which is least sterically hindered, will be abstracted to form the major product.
The complete, detailed mechanisms is shown below:

The outcome of any given reaction can be predicted by considering the factors like nature of the leaving group, substrate, strength of the reagent used, solvent, and temperature.
(e)
Interpretation:
The complete, detailed mechanism for the reaction is to be provided and the products are to be predicted including stereochemistry where appropriate. It is be determined whether the reaction will yield exclusively one product or a mixture of products. If the reaction will yield a mixture of products, then the major product is to be determined.
Concept introduction:
In order to predict the outcome of the given reaction, four factors are considered.
First is to determine if the substrate molecule has a suitable leaving group. If the leaving group is suitable, then evaluate the type of carbon bonded to the leaving group. For an
Answer to Problem 9.63P
The complete, detailed mechanism for the reaction and the products (major) including the stereochemistry are given below:
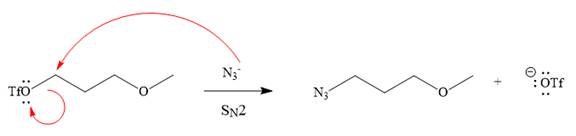
Explanation of Solution
The given reaction is
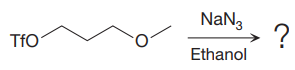
The substrate has one site for substitution or elimination as the
The
The complete, detailed mechanism is shown below:

As the reaction yields a single product, it is the major product.
The outcome of any given reaction can be predicted by considering the factors like nature of the leaving group, substrate, strength of the reagent used, solvent, and temperature.
(f)
Interpretation:
The complete, detailed mechanism for the reaction is to be provided and the products are to be predicted including stereochemistry where appropriate. It is be determined whether the reaction will yield exclusively one product or a mixture of products. If the reaction will yield a mixture of products, then the major product is to be determined.
Concept introduction:
In order to predict the outcome of the given reaction, four factors are considered.
First is to determine if the substrate molecule has a suitable leaving group. If the leaving group is suitable, then evaluate the type of carbon bonded to the leaving group. For an
Answer to Problem 9.63P
The complete, detailed mechanism for the reaction and the products (major) including the stereochemistry are given below:
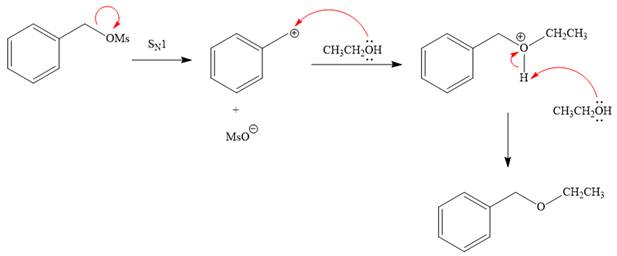
Explanation of Solution
The given reaction is
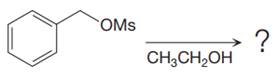
The substrate has one site for substitution or elimination, which is the
The attacking species and the solvent for the reaction is the same, that is, ethanol, which serves as a weak nucleophile and a polar protic solvent. This strongly favors
The first step in the
The complete, detailed mechanism is shown below:
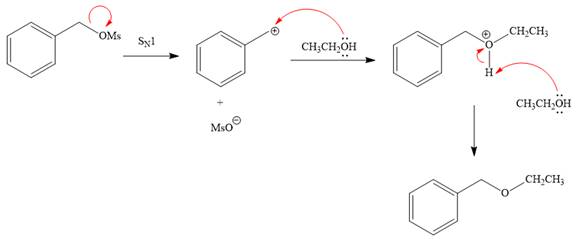
The outcome of any given reaction can be predicted by considering the factors like nature of the leaving group, substrate, strength of the reagent used, solvent, and temperature.
Want to see more full solutions like this?
Chapter 9 Solutions
EBK GET READY FOR ORGANIC CHEMISTRY
- 6. For each of the following, fill in the synthesis arrows with reagents and show the intermediates. You DO NOT need to use the same number of arrows that are shown (you may use more or less), but the product must be formed from the reactant. Then write the mechanism of one step in the synthesis (you can choose which step to write the mechanism for), including all reagents required, clearly labeling the nucleophile and electrophile for each step, and using curved arrows to show the steps in the mechanism. a. b. OHarrow_forwardDraw the productsarrow_forwardDraw the correct productsarrow_forward
- E Organic Chemistry Maxwell Draw the correct products, in either order, for the ozonolysis reaction: 1) O3, CH2Cl2, -78 °C Product 1 + Product 2 2) Zn, HOAc Draw product 1. Select Draw Templates More C H O presented by M Draw product 2. Erase Select Draw Templates M / # # carrow_forward✓ edict the products of this organic reaction: ---- ။ A CH3–C−NH–CH2–C−CH3 + KOH ? Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. If there's more than one product, draw them in any arrangement you like, so long as they aren't touching. If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. Explanation Check Click anywhere to draw the first atom of your structure. C 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibiliarrow_forwardPredict the product of this organic reaction: A HO-C-CH3 + CH3NH2 P+ H2O Specifically, in the drawing area below draw the condensed structure of P. If there is no reasonable possibility for P, check the No answer box under the drawing area. Explanation Check Click anywhere to draw the first atom of your structure. marrow_forward
- H 1) OsO4, pyridine 2) Na2SO3 or NaHSO3 in H₂O 2 productsarrow_forward● Biological Macromolecules Naming and drawing cyclic monosaccharides Your answer is incorrect. • Row 1: Your answer is incorrect. Row 3: Your answer is incorrect. • Row 4: Your answer is incorrect. Try again... 0/5 Give the complete common name, including anomer and stereochemistry labels, of the following molecules. You will find helpful information in the ALEKS resource. CH2OH OH OH H H I H OH OH H] H CH2OH H OH ẞ-L-sorbose HOCH2 OH OH H HOCH2 H OH OH H OH H H CH2OH OH H H OH H I- H OH H OH Explanation Recheck W E R % 25 α B Y X & 5 D F G H McGraw Hill LLC. All Rights Reserved. Terms of Use | Pr Parrow_forwardWhat is the missing reactant in this organic reaction? + R -A HO IN + H₂O Specifically, in the drawing area below draw the skeletal ("line") structure of R. If there is more than one reasonable answer, you can draw any one of them. If there is no reasonable answer, check the No answer box under the drawing area. Note for advanced students: you may assume no products other than those shown above are formed. Explanation Check Click and drag to start drawing a structure. © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Centerarrow_forward
- Stuc X ctclix ALE X A ALE אן A ALEX Lab (195 X Nut x M Inb x NU X NUT X Unt x + → C www-awu.aleks.com/alekscgi/x/Isl.exe/10_u-lgNslkr7j8P3jH-IQ1g8NUi-mObKa_ZLx2twjEhK7mVG6PulJI006NcKTV37JxMpZuyrVCdQolLAKqp_7U3r1GUD3... New Chrome available: Naomi Question 26 of 39 (4 points) | Question Attempt: 1 of Unlimited Give the IUPAC name. 2,3-dimethylhexane Part: 1/2 Part 2 of 2 Draw the skeletal structure of a constitutional isomer of the alkane above that contains a different number of carbons in its longest chain. Skip Part Check Click and drag to start drawing a structure. 3 Finance headline Q Search mwa Harvard Intensifi... X Save For Later 00 dlo HB Submit Assignment 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility a 9:11 PM 4/22/2025arrow_forwardPredict the product of this organic reaction: + NH2 HO A P+ H2O Specifically, in the drawing area below draw the skeletal ("line") structure of P. If there is no reasonable possibility for P, check the No answer box under the drawing area. Click and drag to start drawing a structure. ✓arrow_forward个 Stuc X ctclix ALE X A ALE × A ALE X Lab x (195 × Nut x M Inbx EF 目 → C www-awu.aleks.com/alekscgi/x/Isl.exe/10_u-IgNslkr7j8P3jH-IQ1g8NUi-mObKa_ZLx2twjEhK7mVG6PulJI006NcKTV37JxMpz Chapter 12 HW = Question 27 of 39 (5 points) | Question Attempt: 1 of Unlimited Part: 1/2 Part 2 of 2 Give the IUPAC name. Check 3 50°F Clear ©2025 McGraw Hill L Q Search webp a عالياكarrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

