
Concept explainers
(a)
Interpretation:
Which out of the pair
Concept introduction:
The strength of a nucleophile depends on several factors. In comparison with an uncharged species, a negatively charged species is a stronger nucleophile. In the charged nucleophile, the less stable the charge, the higher the strength as a nucleophile. A negative charge is least stable on a relatively less electronegative atom. Therefore, when comparing atoms from the same period, the less electronegative atom (with a negative charge) is a stronger nucleophile. For atoms from the same group, stability of the charge depends on the size of the atom. If the atom bearing the charge is small, the stability is less and strength as a nucleophile is higher. Resonance can stabilize a charge through delocalization over more than one atom, so resonance stabilization of the charge reduces the strength of a nucleophile. The strength also depends, to some extent, on the inductive effect of the groups attached to the carbon that is bonded to the nucleophilic atom. Inductively electron donating groups destabilize the charge and increase the strength of the nucleophile. On the other hand, inductively electron withdrawing groups decrease the strength of a nucleophile.
Protic solvents strongly solvate and stabilize negatively charged species, and therefore, reduce the strength of negatively charged nucleophiles. In aprotic solvents, solvation is not strong, and they do not significantly affect the strength of a nucleophile.
Answer to Problem 9.56P
Explanation of Solution
Acetone is an aprotic solvent and will not have a significant effect on the strength of the nucleophiles.
The strength of a nucleophile depends on the charge. A negatively charged nucleophile is stronger than an uncharged nucleophile because the charge makes it unstable.
Of the two nucleophiles
Therefore,
A negatively charged nucleophile is stronger than a corresponding uncharged nucleophile.
(b)
Interpretation:
Which of the pair
Concept introduction:
The strength of a nucleophile depends on several factors. A negatively charged species is a stronger nucleophile than an uncharged species, which in turn is stronger than a positively charged species. In a charged nucleophile, the less stable the charge, the higher the strength as a nucleophile. A negative charge is least stable on a relatively less electronegative atom. Therefore, when comparing atoms from the same period, the less electronegative atom (with a negative charge) is a stronger nucleophile. For atoms from the same group, stability of the charge depends on the size of the atom. If the atom bearing the charge is small, the stability is less and strength as a nucleophile is higher. Resonance can stabilize a charge through delocalization over more than one atom, so resonance stabilization of the charge reduces the strength of a nucleophile. The strength also depends, to some extent, on the inductive effect of the groups attached to the carbon that is bonded to the nucleophilic atom. Inductively electron donating groups destabilize the charge and increase the strength of the nucleophile. On the other hand, inductively electron withdrawing groups decrease the strength of a nucleophile.
Answer to Problem 9.56P
From the given pair
Explanation of Solution
Acetone is an aprotic solvent, and will not have a significant effect on the strength of the nucleophiles.
The strength of a nucleophile depends on the charge. An overall positive charge makes it difficult to donate electrons, so a positively charged species is a very weak nucleophile. A negatively charged species is the strongest type of nucleophile.
Therefore, out of the pair
Negatively charged species are strong nucleophiles.
(c)
Interpretation:
Which of the pair
Concept introduction:
The strength of a nucleophile depends on several factors. In comparison with an uncharged species, a negatively charged species is a stronger nucleophile. In a charged nucleophile, the less stable the charge, the higher the strength as a nucleophile. A negative charge is least stable on a relatively less electronegative atom. Therefore, when comparing atoms from the same period, the less electronegative atom (with a negative charge) is a stronger nucleophile. For atoms from the same group, stability of the charge depends on the size of the atom. If the atom bearing the charge is small, the stability is less and strength as a nucleophile is higher. Resonance can stabilize a charge through delocalization over more than one atom, so resonance stabilization of the charge reduces the strength of a nucleophile. The strength also depends, to some extent, on the inductive effect of the groups attached to the carbon that is bonded to the nucleophilic atom. Inductively electron donating groups destabilize the charge and increase the strength of the nucleophile. On the other hand, inductively electron withdrawing groups decrease the strength of a nucleophile.
Protic solvents strongly solvate and stabilize negatively charged species, and therefore, reduce the strength of negatively charged nucleophiles. In aprotic solvents, solvation is not strong, and they do not significantly affect the strength of a nucleophile.
Answer to Problem 9.56P
Explanation of Solution
Acetone is an aprotic solvent and will not have a significant effect on the strength of the nucleophiles.
When the donor atoms are different, the strength depends on the ability of the atom to donate its lone pair. This depends on the electronegativity of the atom when the atoms are from the same period. The electronegativity order for the second period is
Therefore, of the given pair,
Nucleophiles in which the donor atom is relatively less electronegative are stronger nucleophiles than ones with a highly electronegative donor atom.
(d)
Interpretation:
Which of the given pair is the stronger nucleophile in acetone is to be determined.
Concept introduction:
The strength of a nucleophile depends on several factors. In comparison with an uncharged species, a negatively charged species is a stronger nucleophile. In a charged nucleophile, the less stable the charge, the higher the strength as a nucleophile. A negative charge is least stable on a relatively less electronegative atom. Therefore, when comparing atoms from the same period, the less electronegative atom (with a negative charge) is a stronger nucleophile. For atoms from the same group, stability of the charge depends on the size of the atom. If the atom bearing the charge is small, the stability is less and strength as a nucleophile is higher. Resonance can stabilize a charge through delocalization over more than one atom, so resonance stabilization of the charge reduces the strength of a nucleophile. The strength also depends, to some extent, on the inductive effect of the groups attached to the carbon that is bonded to the nucleophilic atom. Inductively electron donating groups destabilize the charge and increase the strength of the nucleophile. On the other hand, inductively electron withdrawing groups decrease the strength of a nucleophile.
Protic solvents strongly solvate and stabilize negatively charged species, and therefore, reduce the strength of negatively charged nucleophiles. In aprotic solvents, solvation is not strong, and they do not significantly affect the strength of a nucleophile.
Answer to Problem 9.56P
The stronger nucleophile from the given pair is
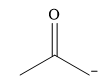
Explanation of Solution
The given pair of nucleophiles is
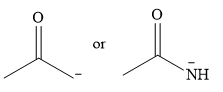
Acetone is an aprotic solvent and will not have a significant effect on the strength of the nucleophiles. The strength will then depend on the nature of the donor atom, the one carrying the negative charge.
When the donor atoms are different, the strength depends on the ability of the atom to donate its lone pair. This depends on the electronegativity of the atom when the atoms are from the same period. The electronegativity order for the second period is
Therefore, of the given pair, the stronger nucleophile in acetone is

Nucleophiles in which the donor atom is relatively less electronegative are stronger nucleophiles than ones with a highly electronegative donor atom.
(e)
Interpretation:
Which of the given pair is the stronger nucleophile in acetone is to be determined.
Concept introduction:
The strength of a nucleophile depends on several factors. In comparison with an uncharged species, a negatively charged species is a stronger nucleophile. In a charged nucleophile, the less stable the charge, the higher the strength as a nucleophile. A negative charge is least stable on a relatively less electronegative atom. Therefore, when comparing atoms from the same period, the less electronegative atom (with a negative charge) is a stronger nucleophile. For atoms from the same group, stability of the charge depends on the size of the atom. If the atom bearing the charge is small, the stability is less and strength as a nucleophile is higher. Resonance can stabilize a charge through delocalization over more than one atom, so resonance stabilization of the charge reduces the strength of a nucleophile. The strength also depends, to some extent, on the inductive effect of the groups attached to the carbon that is bonded to the nucleophilic atom. Inductively electron donating groups destabilize the charge and increase the strength of the nucleophile. On the other hand, inductively electron withdrawing groups decrease the strength of a nucleophile. Protic solvents strongly solvate and stabilize negatively charged species, and therefore, reduce the strength of negatively charged nucleophiles. In aprotic solvents, solvation is not strong, and they do not significantly affect the strength of a nucleophile.
Answer to Problem 9.56P
The stronger nucleophile of the given pair is
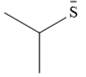
Explanation of Solution
The given pair of nucleophiles is
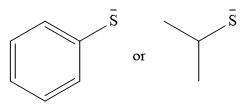
Acetone is an aprotic solvent and will not have a significant effect on the strength of the nucleophiles.
In both the nucleophiles, the donor atom (S) as well as the charge is the same. In this case, the strength will depend on factors such as resonance stabilization and to a lesser extent on inductive effect of the substituents on the carbon attached to the donor atom.
In the first nucleophile, the negative charge on the sulfur atom is adjacent to an
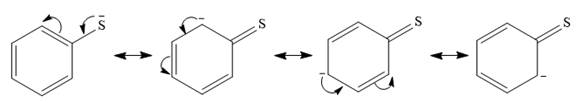
This delocalization will considerably stabilize the negative charge and reduce the ability of sulfur to donate its lone pair. There is no resonance stabilization of the charge on the second nucleophile.
Therefore, the stronger nucleophile of the pair is
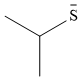
Resonance stabilization of charge reduces the strength of a negatively charged nucleophile.
(f)
Interpretation:
Which of the given pair is the stronger nucleophile in acetone is to be determined.
Concept introduction:
The strength of a nucleophile depends on several factors. In comparison with an uncharged species, a negatively charged species is a stronger nucleophile. In the charged nucleophile, the least stable the charge, the higher the strength as a nucleophile. A negative charge is less stable on a relatively less electronegative atom. Therefore, when comparing atoms from the same period, the less electronegative atom (with a negative charge) is a stronger nucleophile. For atoms from the same group, stability of the charge depends on the size of the atom. If the atom bearing the charge is small, the stability is less and strength as a nucleophile is higher. Resonance can stabilize a charge through delocalization over more than one atom, so resonance stabilization of the charge reduces the strength of a nucleophile. The strength also depends, to some extent, on the inductive effect of the groups attached to the carbon that is bonded to the nucleophilic atom. Inductively electron donating groups destabilize the charge and increase the strength of the nucleophile. On the other hand, inductively electron withdrawing groups decrease the strength of a nucleophile. Protic solvents strongly solvate and stabilize negatively charged species, and therefore, reduce the strength of negatively charged nucleophiles. In aprotic solvents, solvation is not strong, and they do not significantly affect the strength of a nucleophile.
Answer to Problem 9.56P
The stronger nucleophile is
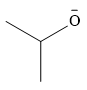
Explanation of Solution
The given pair of nucleophiles is
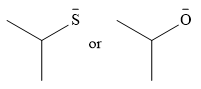
Acetone is an aprotic solvent and will not have a significant effect on the strength of the nucleophiles.
The strength of a nucleophile depends on the charge stability. The one in which the charge stability is less will be the stronger nucleophile.
In the given pair of nucleophiles, the donor atoms are from the same group, and both carrying a full negative charge. The nucleophile strength will then depend on the ability of each atom to stabilize the negative charge. The sulfur atom is considerably larger than the oxygen atom, so the charge density will be less on sulfur than on oxygen. The charge is, therefore, less stable on the oxygen atom.
Therefore, the stronger nucleophile is the oxygen donor nucleophile

In negatively charged nucleophiles, a smaller donor atom stabilizes the charge less, and consequently, is a stronger nucleophile.
(g)
Interpretation:
Which of the given pair is the stronger nucleophile in acetone is to be determined.
Concept introduction:
The strength of a nucleophile depends on several factors. In comparison with an uncharged species, a negatively charged species is a stronger nucleophile. In a charged nucleophile, the less stable the charge, the higher the strength as a nucleophile. A negative charge is least stable on a relatively less electronegative atom. Therefore, when comparing atoms from the same period, the less electronegative atom (with a negative charge) is a stronger nucleophile. For atoms from the same group, stability of the charge depends on the size of the atom. If the atom bearing the charge is small, the stability is less and strength as a nucleophile is higher. Resonance can stabilize a charge through delocalization over more than one atom, so resonance stabilization of the charge reduces the strength of a nucleophile. The strength also depends, to some extent, on the inductive effect of the groups attached to the carbon that is bonded to the nucleophilic atom. Inductively electron donating groups destabilize the charge and increase the strength of the nucleophile. On the other hand, inductively electron withdrawing groups decrease the strength of a nucleophile. Protic solvents strongly solvate and stabilize negatively charged species, and therefore, reduce the strength of negatively charged nucleophiles. In aprotic solvents, solvation is not strong, and they do not significantly affect the strength of a nucleophile.
Answer to Problem 9.56P
The stronger nucleophile of the given pair is
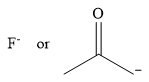
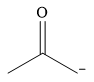
Explanation of Solution
The given pair of nucleophiles is

Acetone is an aprotic solvent and will not have a significant effect on the strength of the nucleophiles.
The two nucleophiles contain donor atoms from the same period, and they have the same charge. The stronger nucleophile will then be the one in which the donor atom is less electronegative.
Therefore, the stronger nucleophile from the given pair is

In negatively charged nucleophiles, the strength increases with decreasing electronegativity of the donor atom, the one carrying the negative charge.
(h)
Interpretation:
Which of the given pair is the stronger nucleophile in acetone is to be determined.
Concept introduction:
The strength of a nucleophile depends on several factors. In comparison with an uncharged species, a negatively charged species is a stronger nucleophile. In the charged nucleophile, the less stable the charge, the higher the strength as a nucleophile. A negative charge is least stable on a relatively less electronegative atom. Therefore, when comparing atoms from the same period, the less electronegative atom (with a negative charge) is a stronger nucleophile. For atoms from the same group, stability of the charge depends on the size of the atom. If the atom bearing the charge is small, the stability is less and strength as a nucleophile is higher. Resonance can stabilize a charge through delocalization over more than one atom, so resonance stabilization of the charge reduces the strength of a nucleophile. The strength also depends, to some extent, on the inductive effect of the groups attached to the carbon that is bonded to the nucleophilic atom. Inductively electron donating groups destabilize the charge and increase the strength of the nucleophile. On the other hand, inductively electron withdrawing groups decrease the strength of a nucleophile. Protic solvents strongly solvate and stabilize negatively charged species, and therefore, reduce the strength of negatively charged nucleophiles. In aprotic solvents, solvation is not strong, and they do not significantly affect the strength of a nucleophile.
Answer to Problem 9.56P
The stronger nucleophile of the two is
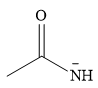
Explanation of Solution
The given pair of nucleophiles is
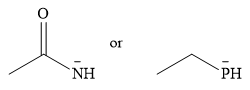
Acetone is an aprotic solvent and will not have a significant effect on the strength of the nucleophiles.
Both are negatively charged nucleophiles. The donor atoms that carry the charge in the two nucleophiles are from the same group. Therefore, the ability of the atom to stabilize the charge will depend on its size. The nucleophile with the smaller donor atom, N, will stabilize the charge less than the one with the larger P donor atom.
Therefore, the stronger nucleophile of the two is

In negatively charged nucleophiles, the strength increases as the charge stability decreases.
(i)
Interpretation:
Which of the pair
Concept introduction:
The strength of a nucleophile depends on several factors. In comparison with an uncharged species, a negatively charged species is a stronger nucleophile. In a charged nucleophile, the less stable the charge, the higher the strength as a nucleophile. A negative charge is least stable on a relatively less electronegative atom. Therefore, when comparing atoms from the same period, the less electronegative atom (with a negative charge) is a stronger nucleophile. For atoms from the same group, stability of the charge depends on the size of the atom. If the atom bearing the charge is small, the stability is less and strength as a nucleophile is higher. Resonance can stabilize a charge through delocalization over more than one atom, so resonance stabilization of the charge reduces the strength of a nucleophile. The strength also depends, to some extent, on the inductive effect of the groups attached to the carbon that is bonded to the nucleophilic atom. Inductively electron donating groups destabilize the charge and increase the strength of the nucleophile. On the other hand, inductively electron withdrawing groups decrease the strength of a nucleophile. Protic solvents strongly solvate and stabilize negatively charged species, and therefore, reduce the strength of negatively charged nucleophiles. In aprotic solvents, solvation is not strong, and they do not significantly affect the strength of a nucleophile.
Answer to Problem 9.56P
The stronger nucleophile of the two is
Explanation of Solution
Acetone is an aprotic solvent and will not have a significant effect on the strength of the nucleophiles.
Both are negatively charged nucleophiles. The donor atoms that carry the charge in the two nucleophiles are from the same group. Therefore, the ability of the atom to stabilize the charge will depend on its size. The nucleophile with the smaller donor atom, S, will stabilize the charge less than the one with the larger donor atom, Se.
Therefore, the stronger nucleophile of the two is
In negatively charged nucleophiles, the strength increases as the charge stability decreases.
(j)
Interpretation:
Which of the pair
Concept introduction:
The strength of a nucleophile depends on several factors. In comparison with an uncharged species, a negatively charged species is a stronger nucleophile. In the charged nucleophile, the less stable the charge, the higher the strength as a nucleophile. A negative charge is less stable on a relatively less electronegative atom Therefore, when comparing atoms from the same period, the less electronegative atom (with a negative charge) is a stronger nucleophile. For atoms from the same group, stability of the charge depends on the size of the atom. If the atom bearing the charge is small, the stability is less and strength as a nucleophile is higher. Resonance can stabilize a charge through delocalization over more than one atom, so resonance stabilization of the charge reduces the strength of a nucleophile. The strength also depends, to some extent, on the inductive effect of the groups attached to the carbon that is bonded to the nucleophilic atom. Inductively electron donating groups destabilize the charge and increase the strength of the nucleophile. On the other hand, inductively electron withdrawing groups decrease the strength of a nucleophile. Protic solvents strongly solvate and stabilize negatively charged species, and therefore, reduce the strength of negatively charged nucleophiles. In aprotic solvents, solvation is not strong, and they do not significantly affect the strength of a nucleophile.
Answer to Problem 9.56P
The stronger nucleophile of the two is
Explanation of Solution
Acetone is an aprotic solvent and will not have a significant effect on the strength of the nucleophiles.
The strength of a nucleophile depends on charge stabilization. The donor atoms, that carry the negative charge, are from the same period. In this case, the less electronegative the atom, lower the stability of the charge, and stronger the corresponding nucleophile. Se is less electronegative than Br.
Therefore,
In negatively charged nucleophiles, the lower the charge stability, the stronger the nucleophile.
Want to see more full solutions like this?
Chapter 9 Solutions
EBK ORGANIC CHEMISTRY: PRINCIPLES AND M
- K Draw the starting structure that would lead to the major product shown under the provided conditions. Drawing 1. NaNH2 2. PhCH2Br 4 57°F Sunny Q Searcharrow_forward7 Draw the starting alkyl bromide that would produce this alkyne under these conditions. F Drawing 1. NaNH2, A 2. H3O+ £ 4 Temps to rise Tomorrow Q Search H2arrow_forward7 Comment on the general features of the predicted (extremely simplified) ¹H- NMR spectrum of lycopene that is provided below. 00 6 57 PPM 3 2 1 0arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

