
Concept explainers
(a)
Interpretation:
The conversion of given starting material into the desired product has to be shown.
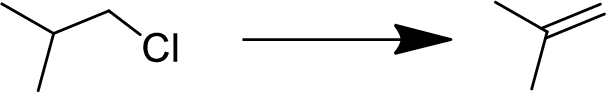
Concept Introduction:
Elimination:
An atom or group are removed from saturated compound to give unsaturated
In elimination, the removal of halogen ion forms a carbocation followed by removal of hydrogen ion forms an alkene is known as E1 reaction.
The abstraction of proton and removal of leaving group takes simultaneously means it is E2 reaction because the
E1 elimination fallows Zaitsev rule (more substituted alkene is formed).
(b)
Interpretation:
The conversion of given starting material into the desired product has to be shown.
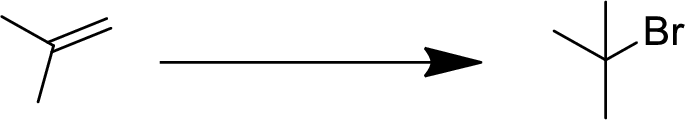
Concept Introduction:
Addition reaction:
Addition of atom or group in carbon–carbon double bond is known as addition reaction.
Markovnikov Rule:
The product of addition reaction is predicted by Markovnikov rule, it state that the negative part of HX is added in the less substituted carbon of alkene.
(c)
Interpretation:
The conversion of given starting material into the desired product has to be shown.

Concept Introduction:
Elimination:
An atom or group are removed from saturated compound to give unsaturated alkene is known as elimination reaction.
In elimination, the removal of halogen ion forms a carbocation followed by removal of hydrogen ion forms an alkene is known as E1 reaction.
The abstraction of proton and removal of leaving group takes simultaneously means it is E2 reaction because the rate of reaction depends on both base and substrate.
E1 elimination fallows Zaitsev rule (more substituted alkene is formed).
Addition reaction:
Addition of atom or group in carbon–carbon double bond is known as addition reaction.
Markovnikov Rule:
The product of addition reaction is predicted by Markovnikov rule, it state that the negative part of HX is added in the less substituted carbon of alkene.
(d)
Interpretation:
The conversion of given starting material into the desired product has to be shown.
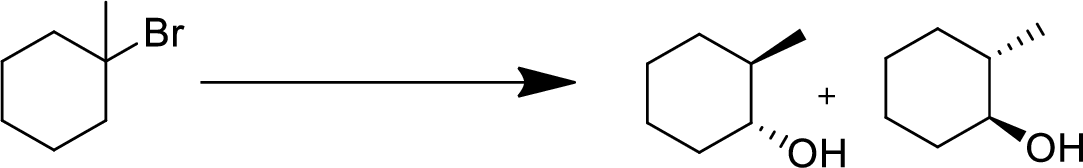
Concept Introduction:
Elimination:
An atom or group are removed from saturated compound to give unsaturated alkene is known as elimination reaction.
In elimination, the removal of halogen ion forms a carbocation followed by removal of hydrogen ion forms an alkene is known as E1 reaction.
The abstraction of proton and removal of leaving group takes simultaneously means it is E2 reaction because the rate of reaction depends on both base and substrate.
E1 elimination fallows Zaitsev rule (more substituted alkene is formed).
Addition reaction:
Addition of atom or group in carbon–carbon double bond is known as addition reaction.
Markovnikov Rule:
The product of addition reaction is predicted by Markovnikov rule, it state that the negative part of HX is added in the less substituted carbon of alkene.
(e)
Interpretation:
The conversion of given starting material into the desired product has to be shown.
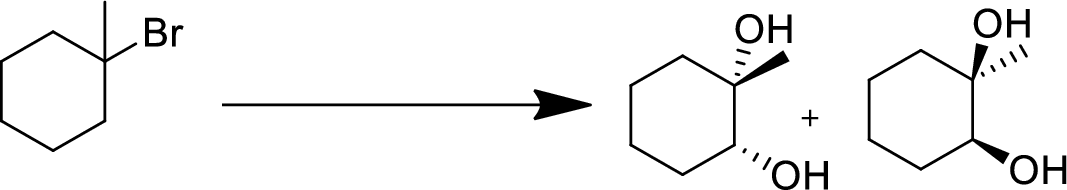
Concept Introduction:
Elimination:
An atom or group are removed from saturated compound to give unsaturated alkene is known as elimination reaction.
In elimination, the removal of halogen ion forms a carbocation followed by removal of hydrogen ion forms an alkene is known as E1 reaction.
The abstraction of proton and removal of leaving group takes simultaneously means it is E2 reaction because the rate of reaction depends on both base and substrate.
E1 elimination fallows Zaitsev rule (more substituted alkene is formed).
Addition reaction:
Addition of atom or group in carbon–carbon double bond is known as addition reaction.
Markovnikov Rule:
The product of addition reaction is predicted by Markovnikov rule, it state that the negative part of HX is added in the less substituted carbon of alkene.
(f)
Interpretation:
The conversion of given starting material into the desired product has to be shown.
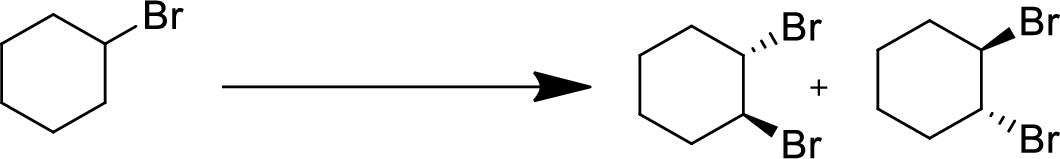
Concept Introduction:
Elimination:
An atom or group are removed from saturated compound to give unsaturated alkene is known as elimination reaction.
In elimination, the removal of halogen ion forms a carbocation followed by removal of hydrogen ion forms an alkene is known as E1 reaction.
The abstraction of proton and removal of leaving group takes simultaneously means it is E2 reaction because the rate of reaction depends on both base and substrate.
E1 elimination fallows Zaitsev rule (more substituted alkene is formed).
Addition reaction:
Addition of atom or group in carbon–carbon double bond is known as addition reaction.
Markovnikov Rule:
The product of addition reaction is predicted by Markovnikov rule, it state that the negative part of HX is added in the less substituted carbon of alkene.
(g)
Interpretation:
The conversion of given starting material into the desired product has to be shown.

Concept Introduction:
(h)
Interpretation:
The conversion of given starting material into the desired product has to be shown.
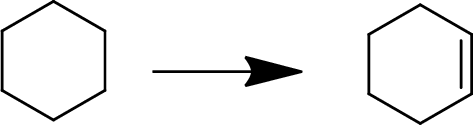
Concept Introduction:
Elimination:
An atom or group are removed from saturated compound to give unsaturated alkene is known as elimination reaction.
In elimination, the removal of halogen ion forms a carbocation followed by removal of hydrogen ion forms an alkene is known as E1 reaction.
The abstraction of proton and removal of leaving group takes simultaneously means it is E2 reaction because the rate of reaction depends on both base and substrate.
E1 elimination fallows Zaitsev rule (more substituted alkene is formed).
(i)
Interpretation:
The conversion of given starting material into the desired product has to be shown.
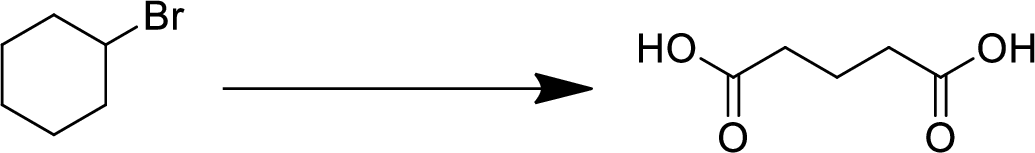
Concept Introduction:
Elimination:
An atom or group are removed from saturated compound to give unsaturated alkene is known as elimination reaction.
In elimination, the removal of halogen ion forms a carbocation followed by removal of hydrogen ion forms an alkene is known as E1 reaction.
The abstraction of proton and removal of leaving group takes simultaneously means it is E2 reaction because the rate of reaction depends on both base and substrate.
E1 elimination fallows Zaitsev rule (more substituted alkene is formed).
Trending nowThis is a popular solution!

Chapter 9 Solutions
OWLv2 with MindTap Reader, 1 term (6 months) Printed Access Card for Brown/Iverson/Anslyn/Foote's Organic Chemistry, 8th Edition
- A 0.552-g sample of an unknown acid was dissolved in water to a total volume of 20.0 mL. This sample was titrated with 0.1103 M KOH. The equivalence point occurred at 29.42 mL base added. The pH of the solution at 10.0 mL base added was 3.72. Determine the molar mass of the acid. Determine the Ka of the acid.arrow_forwardAs the lead product manager at OrganometALEKS Industries, you are trying to decide if the following reaction will its major product: 2,0° with a new C-C bond as If this reaction will work, draw the major organic product or products you would expect in the drawing aree below. If there's more than one major product, you can draw them in any arrangement you like. Be sure you use wedge and desh bonds if necessary, for example to distinguish between major products with different stereochemistry. If the major products of this reaction won't have a new C-C bond, just check the box under the drawing area and leave it blank.arrow_forwardwrite the mechanism of the nucleophilic acyl substitution reaction, please give an examplearrow_forward
- The compound in the figure is reacted with 10 n-butyllihium, 2° propanone, and 3º H2O. Draw and name the products obtained. SiMe3arrow_forwardCaffeine (C8H10N4O2, pictured below) is a weak base. The pKb of caffeine is 10.4. What is the pH of a 0.0155 M solution of caffeine?arrow_forward2-Cyclopentyl-2-methyl-1,3-dioxolane is reacted with H₂SO₄. Draw and name the structures of the products.arrow_forward
- Indicate the products of the reaction of 1-cyclohexyl-2,2-dimethylpropan-1-one with CH3CO3H (). Draw the structures of the compounds.arrow_forwardWrite chemical equations for: the reaction of benzoic acid chloride with grignard reagent [CH3MgX] the reaction of butanoic acid with methyl amine [CH3NH2]arrow_forward2-(3-Aminopropyl)cyclohexan-1-one is reacted with H₂SO₄. Draw the structures of the products.arrow_forward
- Please help me solve number 2arrow_forwardChoose the best reagents to complete the following reaction. 오 Na2Cr2O7 H2SO4, H2O Problem 22 of 35 A Na2Cr2O7 H2SO4, H2O H2/Pt B pressure OH 1. NaBH4 C 2. H3O+ D DMP (Dess-Martin Periodinane) CH2Cl2 CrO3 Done Dramabana_Minor Submitarrow_forwardIndicate the products of the reaction of Cycloheptanone with pyrrolidine (cat. H+). Draw the structures of the compounds.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
