
Concept explainers
a.
Interpretation: The molecules that contain polar bonds; the polar molecules and the non-polar molecules from the given molecules are to be identified.
Concept introduction: Polarity in bond arises due to the difference in electronegativity of the bonded atoms.
If the net dipole moment of the molecule is zero, the molecule is non-polar.
If the molecule has some net dipole moment than the molecule is polar.
The overall polarity of the molecule is determined by calculating the vector sum of the individual bond polarities.
To identify: The molecules that contain polar bonds.
a.
Answer to Problem 9.45QP
Solution
All the molecules contain polar bonds in there structure.
Explanation of Solution
Explanation
Polarity in bond arises due to the difference in electronegativity of the bonded atoms.
For molecule (a)
In 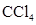 , there is difference in electronegativity of carbon and chlorine atoms because chlorine is more electronegative than carbon.
, there is difference in electronegativity of carbon and chlorine atoms because chlorine is more electronegative than carbon.
Therefore, the bonds in 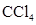 is polar.
is polar.
For molecule (b)
In 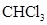 , there is difference in electronegativity of carbon and hydrogen atom because carbon is more electronegative than hydrogen.
, there is difference in electronegativity of carbon and hydrogen atom because carbon is more electronegative than hydrogen.
Therefore, the 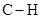 bond in
bond in 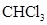 is polar.
is polar.
In 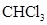 , there is difference in electronegativity of carbon and chlorine atom because chlorine is more electronegative than carbon.
, there is difference in electronegativity of carbon and chlorine atom because chlorine is more electronegative than carbon.
Therefore, the 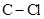 bond in
bond in 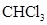 is polar.
is polar.
For molecule (c)
In 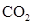 , there is difference in electronegativity of carbon and oxygen atoms because oxygen is more electronegative than carbon.
, there is difference in electronegativity of carbon and oxygen atoms because oxygen is more electronegative than carbon.
Therefore, the 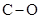 bonds in
bonds in 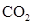 is polar.
is polar.
For molecule (d)
In 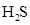 , there is difference in electronegativity of sulphur and hydrogen atom because sulphur is more electronegative than hydrogen.
, there is difference in electronegativity of sulphur and hydrogen atom because sulphur is more electronegative than hydrogen.
Therefore, the 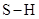 bond in
bond in 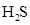 is polar.
is polar.
For molecule (e)
In 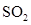 , there is difference in electronegativity of sulphur and oxygen atoms because oxygen is more electronegative than sulphur.
, there is difference in electronegativity of sulphur and oxygen atoms because oxygen is more electronegative than sulphur.
Therefore, the 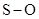 bond in
bond in 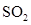 is polar.
is polar.
b.
To identify: The molecules that are polar.
b.
Answer to Problem 9.45QP
Solution
The polar molecules are 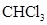 ,
,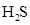 and
and 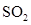 .
.
Explanation of Solution
Explanation
Polarity in bond arises due to the difference in electronegativity of the bonded atoms.
If the net dipole moment of the molecule is zero, the molecule is non polar in nature.
If the molecule has some net dipole moment than the molecule is polar in nature.
The overall polarity of the molecule is determined by calculating the vector sum of the individual bond polarities.
For molecule (a)
In 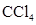 , there is difference in electronegativity of carbon and chlorine atoms because chlorine is more electronegative than carbon.
, there is difference in electronegativity of carbon and chlorine atoms because chlorine is more electronegative than carbon.
Therefore, the bonds in 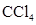 are polar.
are polar.
The dipole moment of 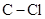 bonds get cancelled out to give zero net dipole moment to the molecule.
bonds get cancelled out to give zero net dipole moment to the molecule.
Therefore, the 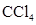 is non polar.
is non polar.
For molecule (b)
In 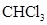 , there is difference in electronegativity of carbon and hydrogen atom because carbon is more electronegative than hydrogen.
, there is difference in electronegativity of carbon and hydrogen atom because carbon is more electronegative than hydrogen.
Therefore, the 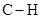 bond in
bond in 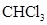 is polar.
is polar.
In 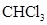 , there is difference in electronegativity of carbon and chlorine atom because chlorine is more electronegative than carbon.
, there is difference in electronegativity of carbon and chlorine atom because chlorine is more electronegative than carbon.
Therefore, the 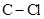 bonds in
bonds in 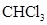 is polar.
is polar.
The sum of dipole moment of one 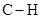 bond and three
bond and three 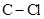 give a net dipole moment to the molecule.
give a net dipole moment to the molecule.
Therefore, the 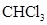 molecule is polar.
molecule is polar.
For molecule (c)
In 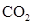 , there is difference in electronegativity of carbon and oxygen atoms because oxygen is more electronegative than carbon.
, there is difference in electronegativity of carbon and oxygen atoms because oxygen is more electronegative than carbon.
Therefore, the 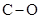 bonds in
bonds in 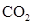 is polar.
is polar.
The dipole moment of 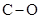 bonds get cancelled out to give zero net dipole moment to the molecule.
bonds get cancelled out to give zero net dipole moment to the molecule.
Therefore, the 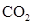 is non polar.
is non polar.
For molecule (d)
In 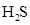 , there is difference in electronegativity of sulphur and hydrogen atom because sulphur is more electronegative than hydrogen.
, there is difference in electronegativity of sulphur and hydrogen atom because sulphur is more electronegative than hydrogen.
Therefore, the 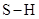 bondS in
bondS in 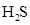 is polar.
is polar.
The sum of dipole moment of two 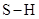 bonds gives a net dipole moment to the molecule.
bonds gives a net dipole moment to the molecule.
Therefore, the 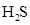 molecule is polar.
molecule is polar.
For molecule (e)
In 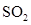 , there is difference in electronegativity of sulphur and oxygen atoms because oxygen is more electronegative than sulphur.
, there is difference in electronegativity of sulphur and oxygen atoms because oxygen is more electronegative than sulphur.
Therefore, the 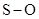 bonds in
bonds in 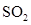 is polar.
is polar.
The structure of 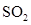 is bent. The sum of dipole moment of two
is bent. The sum of dipole moment of two 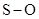 bonds gives a net dipole moment to the molecule.
bonds gives a net dipole moment to the molecule.
Therefore, the 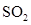 molecule is polar.
molecule is polar.
c.
To identify: The molecules that are non polar.
c.
Answer to Problem 9.45QP
Solution
The polar molecules are 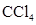 and
and 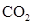 .
.
Explanation of Solution
Explanation
Polarity in bond arises due to the difference in electronegativity of the bonded atoms.
If the net dipole moment of the molecule is zero, the molecule is non polar in nature.
If the molecule has some net dipole moment than the molecule is polar in nature.
The overall polarity of the molecule is determined by calculating the vector sum of the individual bond polarities.
For molecule (a)
In 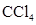 , there is difference in electronegativity of carbon and chlorine atoms because chlorine is more electronegative than carbon.
, there is difference in electronegativity of carbon and chlorine atoms because chlorine is more electronegative than carbon.
Therefore, the bonds in 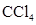 is polar.
is polar.
The dipole moment of 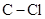 bonds get cancelled out to give zero net dipole moment to the molecule.
bonds get cancelled out to give zero net dipole moment to the molecule.
Therefore, the 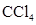 is non polar.
is non polar.
For molecule (b)
In 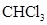 , there is difference in electronegativity of carbon and hydrogen atom because carbon is more electronegative than hydrogen.
, there is difference in electronegativity of carbon and hydrogen atom because carbon is more electronegative than hydrogen.
Therefore, the 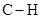 bond in
bond in 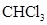 is polar.
is polar.
In 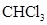 , there is difference in electronegativity of carbon and chlorine atom because chlorine is more electronegative than carbon.
, there is difference in electronegativity of carbon and chlorine atom because chlorine is more electronegative than carbon.
Therefore, the 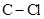 bonds in
bonds in 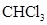 is polar.
is polar.
The sum of dipole moment of one 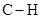 bond and three
bond and three 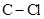 give a net dipole moment to the molecule.
give a net dipole moment to the molecule.
Therefore, the 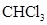 molecule is polar.
molecule is polar.
For molecule (c)
In 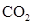 , there is difference in electronegativity of carbon and oxygen atoms because oxygen is more electronegative than carbon.
, there is difference in electronegativity of carbon and oxygen atoms because oxygen is more electronegative than carbon.
Therefore, the 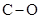 bonds in
bonds in 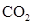 is polar.
is polar.
The dipole moment of 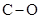 bonds get cancelled out to give zero net dipole moment to the molecule.
bonds get cancelled out to give zero net dipole moment to the molecule.
Therefore, the 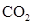 is non polar.
is non polar.
For molecule (d)
In 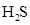 , there is difference in electronegativity of sulphur and hydrogen atom because sulphur is more electronegative than hydrogen.
, there is difference in electronegativity of sulphur and hydrogen atom because sulphur is more electronegative than hydrogen.
Therefore, the 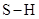 bond in
bond in 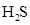 is polar.
is polar.
The sum of dipole moment of two 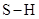 bonds gives a net dipole moment to the molecule.
bonds gives a net dipole moment to the molecule.
Therefore, the 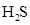 molecule is polar.
molecule is polar.
For molecule (e)
In 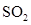 , there is difference in electronegativity of sulphur and oxygen atoms because oxygen is more electronegative than sulphur.
, there is difference in electronegativity of sulphur and oxygen atoms because oxygen is more electronegative than sulphur.
Therefore, the 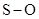 bonds in
bonds in 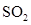 is polar.
is polar.
The structure of 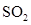 is bent. The sum of dipole moment of two
is bent. The sum of dipole moment of two 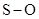 bonds gives a net dipole moment to the molecule.
bonds gives a net dipole moment to the molecule.
Therefore, the 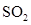 molecule is polar.
molecule is polar.
Conclusion
- a. All the molecules contains polar bonds in there structure.
- b. The polar molecules are
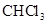 ,
, 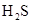 and
and 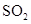 .
. - c. The polar molecules are
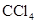 and
and 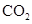 .
.
Want to see more full solutions like this?
Chapter 9 Solutions
Chemistry: The Science in Context (Fourth Edition)
- K Draw the starting structure that would lead to the major product shown under the provided conditions. Drawing 1. NaNH2 2. PhCH2Br 4 57°F Sunny Q Searcharrow_forward7 Draw the starting alkyl bromide that would produce this alkyne under these conditions. F Drawing 1. NaNH2, A 2. H3O+ £ 4 Temps to rise Tomorrow Q Search H2arrow_forward7 Comment on the general features of the predicted (extremely simplified) ¹H- NMR spectrum of lycopene that is provided below. 00 6 57 PPM 3 2 1 0arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





