
Concept explainers
(a)
Interpretation:
The structural formula for the product of given
Concept Introduction:
Structure of the substrate plays a major role in
(a)
Answer to Problem 9.13P
The structural formula for the product of given

Explanation of Solution
In the given
Given reactant is non-chiral so product also non-chiral.

(b)
Interpretation:
The structural formula for the product of given
Concept Introduction:
Structure of the substrate plays a major role in
(b)
Answer to Problem 9.13P
The structural formula for the product of given
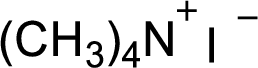
Explanation of Solution
In the given
In this reaction formed quaternary ammonium ion is stabilized by iodine ion so the product is an iodine salt of quaternary ammonium ion.
Given reactant is non-chiral so product also non-chiral.

(c)
Interpretation:
The structural formula for the product of given
Concept Introduction:
Structure of the substrate plays a major role in
(c)
Answer to Problem 9.13P
The structural formula for the product of given
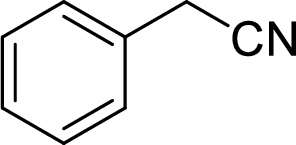
Explanation of Solution
In the given
Given reactant is non-chiral so product also non-chiral.
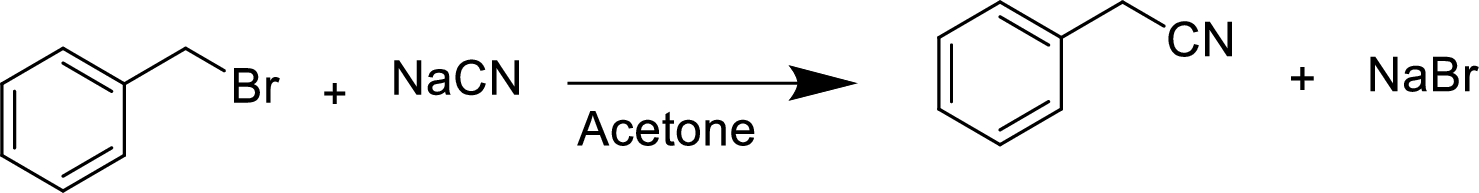
(d)
Interpretation:
The structural formula for the product of given
Concept Introduction:
Structure of the substrate plays a major role in
(d)
Answer to Problem 9.13P
The structural formula for the product of given
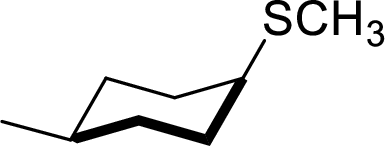
Explanation of Solution
In the given
Given reactant has an equatorial chlorine hence, the product is axial.
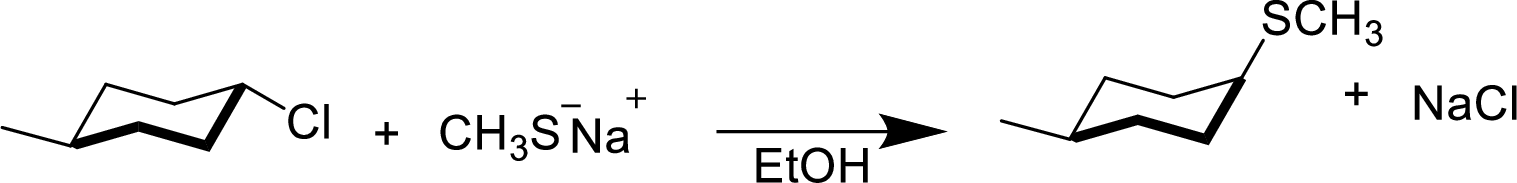
(e)
Interpretation:
The structural formula for the product of given
Concept Introduction:
Structure of the substrate plays a major role in
(e)
Answer to Problem 9.13P
The structural formula for the product of given
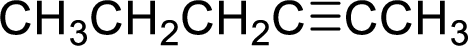
Explanation of Solution
In the given
Given reactant is non-chiral so product also non-chiral.

(f)
Interpretation:
The structural formula for the product of given
Concept Introduction:
Structure of the substrate plays a major role in
(f)
Answer to Problem 9.13P
The structural formula for the product of given
Explanation of Solution
In the given
In this reaction formed ammonium ion is stabilized by chorine ion so the product is a chlorine salt of ammonium ion.
Given reactant is non-chiral so product also non-chiral.
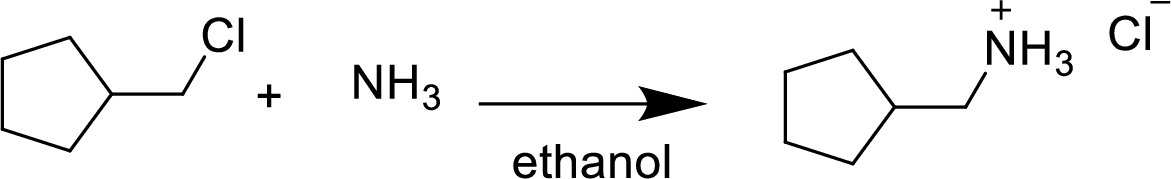
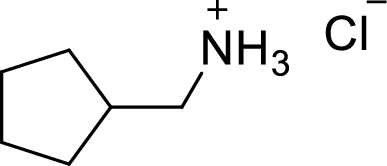
(g)
Interpretation:
The structural formula for the product of given
Concept Introduction:
Structure of the substrate plays a major role in
(g)
Answer to Problem 9.13P
The structural formula for the product of given
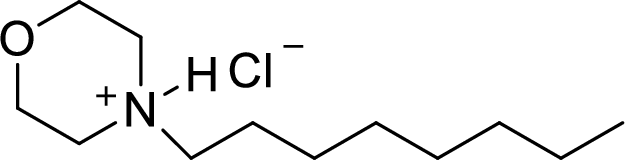
Explanation of Solution
In the given
In this reaction formed ammonium ion is stabilized by chorine ion so the product is a chlorine salt of ammonium ion.
Given reactant is non-chiral so product also non-chiral.

(h)
Interpretation:
The structural formula for the product of given
Concept Introduction:
Structure of the substrate plays a major role in
(h)
Answer to Problem 9.13P
The structural formula for the product of given

Explanation of Solution
In the given
Given reactant is non-chiral so product also non-chiral.

Want to see more full solutions like this?
Chapter 9 Solutions
Organic Chemistry, Loose-leaf Version
- The reaction Q(g) + R(g) → Z(l) is shown to be exothermic. Which of the following is true concerning the reactionarrow_forwardWhich of the following has the largest standard molar entropy, S° (298.15 K) He H2 NaCl KBr Hgarrow_forwardWhich of the following is true for a particular reaction if ∆G° is -40.0 kJ/mol at 290 K and –20.0 kJ/mol at 390 K?arrow_forward
- Choose the major product of the reaction with correct regio- and stereochemistry. Br2 H₂O O "Br Br & O 'Br OH Br 吡 O OH OH Br "OH Brarrow_forwardSelect the major product of the following reaction. & Br (CH)CONa (CH₂),COH 0 OC(CH) O &arrow_forwardDraw the products of the hydrolysis reaction between the ester molecule and water. Determine the products of the following reaction.arrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning



