
Chemistry: A Molecular Approach (4th Edition)
4th Edition
ISBN: 9780134112831
Author: Nivaldo J. Tro
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 9, Problem 72E
Interpretation Introduction
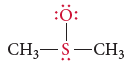
To determine: The formal charges of the atoms shown in red.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
What is the total energy cost associated with the compound below adopting the shown conformation?
CH3
HH
DH
CH3
ΗΝ,
Draw Final Product
C
cyclohexanone
pH 4-5
Edit Enamine
H3O+
CH3CH2Br
THF, reflux
H
Edit Iminium Ion
How many hydrogen atoms are connected to the indicated carbon atom?
Chapter 9 Solutions
Chemistry: A Molecular Approach (4th Edition)
Ch. 9 - Prob. 1SAQCh. 9 - Q2. Which set of elements is arranged in order of...Ch. 9 - Q3. Which is the correct Lewis structure for...Ch. 9 - Q4. Which compound is likely to have an incomplete...Ch. 9 - Prob. 5SAQCh. 9 - Prob. 6SAQCh. 9 - Prob. 7SAQCh. 9 - Prob. 8SAQCh. 9 - Prob. 9SAQCh. 9 - Prob. 10SAQ
Ch. 9 - Q11. Determine the formal charge of nitrogen in...Ch. 9 - Prob. 12SAQCh. 9 - Q13. Use formal charge to choose the best Lewis...Ch. 9 - Prob. 14SAQCh. 9 - Prob. 15SAQCh. 9 - Prob. 1ECh. 9 - Prob. 2ECh. 9 - Prob. 3ECh. 9 - Prob. 4ECh. 9 - 5. Describe the octet rule in the Lewis model.
Ch. 9 - 6. According to the Lewis model, what is a...Ch. 9 - 7. How do you draw an ionic Lewis structure?
Ch. 9 - 8. How can Lewis structures be used to determine...Ch. 9 - Prob. 9ECh. 9 - Prob. 10ECh. 9 - Prob. 11ECh. 9 - Prob. 12ECh. 9 - Prob. 13ECh. 9 - Prob. 14ECh. 9 - 15. In a covalent Lewis structure, what is the...Ch. 9 - Prob. 16ECh. 9 - 17. How does the Lewis model for covalent bonding...Ch. 9 - 18. How does the Lewis model for covalent bonding...Ch. 9 - 19. What is electronegativity? What are the...Ch. 9 - Prob. 20ECh. 9 - 21. Explain percent ionic character of a bond. Do...Ch. 9 - 22. What is a dipole moment?
Ch. 9 - Prob. 23ECh. 9 - Prob. 24ECh. 9 - Prob. 25ECh. 9 - 26. What are resonance structures? What is a...Ch. 9 - 27. Do resonance structures always contribute...Ch. 9 - 28. What is formal charge? How is formal charge...Ch. 9 - 29. Why does the octet rule have exceptions? List...Ch. 9 - 30. Which elements can have expanded octets? Which...Ch. 9 - Prob. 31ECh. 9 - Prob. 32ECh. 9 - 33. What is the electron sea model for bonding in...Ch. 9 - Prob. 34ECh. 9 - 35. Write the electron configuration for N. Then...Ch. 9 - 36. Write the electron configuration for Ne. Then...Ch. 9 - 37. Write the Lewis symbol for each atom or...Ch. 9 - 38. Write the Lewis symbol for each atom or...Ch. 9 - 39. Write the Lewis symbols for the ions in each...Ch. 9 - 40. Write the Lewis symbols for the ions in each...Ch. 9 - 41. Use Lewis symbols to determine the formula for...Ch. 9 - 42. Use Lewis symbols to determine the formula for...Ch. 9 - 43. Explain the trend in the lattice energies of...Ch. 9 - 44. Rubidium iodide has a lattice energy of –617...Ch. 9 - 45. The lattice energy of CsF is –744 kJ/mol,...Ch. 9 - 46. Arrange these compounds in order of increasing...Ch. 9 - 47. Use the Born–Haber cycle and data from...Ch. 9 - 48. Use the Born–Haber cycle and data from...Ch. 9 - 49. Use covalent Lewis structures to explain why...Ch. 9 - 50. Use covalent Lewis structures to explain why...Ch. 9 - 51. Write the Lewis structure for each...Ch. 9 - 52. Write the Lewis structure for each...Ch. 9 - 53. Write the Lewis structure for each...Ch. 9 - 54. Write the Lewis structure for each...Ch. 9 - 55. Determine if a bond between each pair of atoms...Ch. 9 - 56. Determine if a bond between each pair of atoms...Ch. 9 - 57. Draw the Lewis structure for CO with an arrow...Ch. 9 - 58. Draw the Lewis structure for BrF with an arrow...Ch. 9 - 59. Write the Lewis structure for each molecule or...Ch. 9 - 60. Write the Lewis structure for each molecule or...Ch. 9 - 61. Write the Lewis structure for each molecule or...Ch. 9 - 62. Write the Lewis structure for each molecule or...Ch. 9 - 63. Write a Lewis structure that obeys the octet...Ch. 9 - 64. Write a Lewis structure that obeys the octet...Ch. 9 - 65. Use formal charge to identify the better Lewis...Ch. 9 - 66. Use formal charges to identify the better...Ch. 9 - 67. How important is the resonance structure shown...Ch. 9 - 68. In N2O, nitrogen is the central atom and the...Ch. 9 - 69. Draw the Lewis structure (including resonance...Ch. 9 - 70. Draw the Lewis structure (including resonance...Ch. 9 - 71. What are the formal charges of the atoms shown...Ch. 9 - 72. What are the formal charges of the atoms shown...Ch. 9 - 73. Write the Lewis structure for each molecule...Ch. 9 - 74. Write the Lewis structure for each molecule...Ch. 9 - 75. Write the Lewis structure for each ion....Ch. 9 - 76. Write Lewis structures for each molecule or...Ch. 9 - 77. Write Lewis structures for each molecule or...Ch. 9 - 78. Write Lewis structures for each molecule or...Ch. 9 - 79. Order these compounds in order of increasing...Ch. 9 - 80. Which compound shown here has the stronger...Ch. 9 - 81. Hydrogenation reactions are used to add...Ch. 9 - 82. Ethanol is a possible fuel. Use average bond...Ch. 9 - 83. Hydrogen, a potential future fuel, can be...Ch. 9 - Prob. 84ECh. 9 - 85. Write an appropriate Lewis structure for each...Ch. 9 - 86. Write an appropriate Lewis structure for each...Ch. 9 - 87. Each compound contains both ionic and covalent...Ch. 9 - 88. Each compound contains both ionic and covalent...Ch. 9 - 89. Carbon ring structures are common in organic...Ch. 9 - 90. Amino acids are the building blocks of...Ch. 9 - 91. Formic acid is responsible for the sting of...Ch. 9 - 92. Diazomethane is a highly poisonous, explosive...Ch. 9 - 93. The reaction of Fe2O3(s) with Al(s) to form...Ch. 9 - 94. NaCl has a lattice energy of –787 kJ/mol....Ch. 9 - 95. Draw the Lewis structure for nitric acid (the...Ch. 9 - 96. Phosgene (Cl2CO) is a poisonous gas used as a...Ch. 9 - 97. The cyanate ion (OCN–) and the fulminate ion...Ch. 9 - Prob. 98ECh. 9 - Prob. 99ECh. 9 - 100. Use Lewis structures to explain why Br3– and...Ch. 9 - 101. Draw the Lewis structure for HCSNH2. (The...Ch. 9 - 102. Draw the Lewis structure for urea, H2NCONH2,...Ch. 9 - 103. Some theories of aging suggest that free...Ch. 9 - 104. Free radicals are important in many...Ch. 9 - Prob. 105ECh. 9 - 106. Calculate ΔHrxn for the combustion of octane...Ch. 9 - 107. Draw the Lewis structure for each...Ch. 9 - Prob. 108ECh. 9 - Prob. 109ECh. 9 - 110. Calculate for the reaction using the bond...Ch. 9 - Prob. 111ECh. 9 - Prob. 112ECh. 9 - 113. A compound composed of only carbon and...Ch. 9 - Prob. 114ECh. 9 - 115. The main component of acid rain (H2SO4) forms...Ch. 9 - 116. A 0.167-g sample of an unknown acid requires...Ch. 9 - Prob. 117ECh. 9 - Prob. 118ECh. 9 - Prob. 119ECh. 9 - 120. The standard heat of formation of CaBr2 is...Ch. 9 - Prob. 121ECh. 9 - Prob. 122ECh. 9 - Prob. 123ECh. 9 - Prob. 124ECh. 9 - Prob. 125ECh. 9 - 126. Which statement is true of an endothermic...Ch. 9 - Prob. 127ECh. 9 - Prob. 128ECh. 9 - Prob. 129ECh. 9 - Prob. 130QGWCh. 9 - Prob. 131QGWCh. 9 - Prob. 132QGWCh. 9 - 133. Draft a list of step-by-step instructions for...Ch. 9 - Prob. 134QGWCh. 9 - 135. Evidence for the additional stabilization of...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Identify the compound with the longest carbon - nitrogen bond. O CH3CH2CH=NH O CH3CH2NH2 CH3CH2C=N CH3CH=NCH 3 The length of all the carbon-nitrogen bonds are the samearrow_forwardIdentify any polar covalent bonds in epichlorohydrin with S+ and 8- symbols in the appropriate locations. Choose the correct answer below. Η H's+ 6Η Η Η Η Η Ηδ Η Ο Ο HH +Η Η +Η Η Η -8+ CIarrow_forwardH H:O::::H H H HH H::O:D:D:H HH HH H:O:D:D:H .. HH H:O:D:D:H H H Select the correct Lewis dot structure for the following compound: CH3CH2OHarrow_forward
- Rank the following compounds in order of decreasing boiling point. ннннн -С-С-Н . н-с- ННННН H ΗΤΗ НННН TTTĪ н-с-с-с-с-о-н НННН НН C' Н н-с-с-с-с-н НН || Ш НННН H-C-C-C-C-N-H ННННН IVarrow_forwardRank the following compounds in order of decreasing dipole moment. |>||>||| ||>|||>| |>|||>|| |||>||>| O ||>>||| H F H F H c=c || H c=c F F IIIarrow_forwardchoose the description that best describes the geometry for the following charged species ch3-arrow_forward
- Why isn't the ketone in this compound converted to an acetal or hemiacetal by the alcohol and acid?arrow_forwardWhat is the approximate bond angle around the nitrogen atom? HNH H Harrow_forwardOH 1. NaOCH2CH3 Q 2. CH3CH2Br (1 equiv) H3O+ Select to Draw 1. NaOCH2 CH3 2. CH3Br (1 equiv) heat Select to Edit Select to Drawarrow_forward
- Complete and balance the following half-reaction in acidic solution. Be sure to include the proper phases for all species within the reaction. S₂O₃²⁻(aq) → S₄O₆²⁻(aq)arrow_forwardQ Select to Edit NH3 (CH3)2CHCI (1 equiv) AICI 3 Select to Draw cat. H2SO4 SO3 (1 equiv) HO SOCl2 pyridine Select to Edit >arrow_forwardComplete and balance the following half-reaction in basic solution. Be sure to include the proper phases for all species within the reaction. Zn(s) → Zn(OH)₄²⁻(aq)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY