
(a)
The mass of sugar dissolved in
(a)
Answer to Problem 1QAP
The mass of sugar dissolved in
Explanation of Solution
Given Amount of water is
Temperature is
Formula used:
Where,
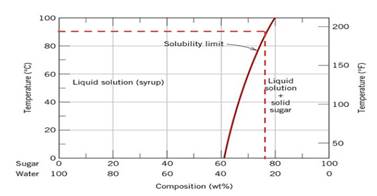
According to diagram
Calculations Put value of
Conclusion So, the mass of sugar dissolved in
(b)
Composition of saturated liquid solution at
(b)
Answer to Problem 1QAP
Composition of the saturated liquid solution is
Explanation of Solution
From the sugar-water phase diagram when the liquid saturated solution from temperature
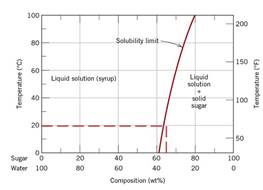
Conclusion Hence, the composition of the saturated liquid solution is
(c)
Mass of solid sugar comes out of the solution upon cooling to
(c)
Answer to Problem 1QAP
Mass of solid sugar comes out of the solution upon cooling to
Explanation of Solution
Given Amount of water is
Cooling Temperature is
Mass of sugar dissolved at
Formula used:
Solubility of sugar in water is given by:
Where,
Mass of sugar solidifies upon cooling to temperature
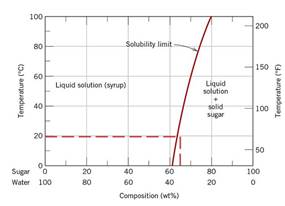
According to diagram
Calculations Put value of
Mass of sugar solidifies upon cooling to temperature
Conclusion So, the mass of sugar that solidified upon cooling to temperature
Want to see more full solutions like this?
Chapter 9 Solutions
MATERIALS SCIENCE AND ENGINEERING -PACK
- 4. Design an operational amplifier circuit to implement the following mathematical equation. 0.25 dv dtt dvo + ·+ V₁ = Vi dtarrow_forwardsolve and show workarrow_forwardProblem 4 Consider a unity (negative) feedback system whose open-loop transfer function is given by K(s+1)(s+2) G(s): s(s +10) Assume K = 1. (a) What is the type of the system? (b) Find static position error constant Kp, static velocity error constant Ky and static acceleration error constant Ka (c) Find the steady state-error of the system for following each of the following inputs. (i) (!!) t³ 1(t) (t+2) 1(t) (d) Find the range of K, for which steady-state error of the system for ramp input will be less than 0.05?arrow_forward
- 4.5m 4.5m 4.5m 20 4m A- Intermediate flat plate floor, story height=2.75 m, t=190 mm, f'c=20 MPa for slabs and f'c=35 MPa for columns. All columns are 400×400mm. Find all DF for the interior equivalent frame shown. 6m 6marrow_forwardAn inner-city metro-bus weighs approximately 10,000 kg including passenger loads, travels 500 km per fully charged battery, and consumes 420 Wh/km. Design a lithium-ion battery pack for the metro-bus using newly developed cells made of silicon anode and lithium manganese-iron phosphate (LMFP) with formulation of Si // 4(LiMn5Fe0.5PO4). The cell average voltage is 3.5V and its capacity 4Ah. The nominal battery pack voltage is 350V. Report the battery pack configuration: Calculate the amount of silicon and LMFP cathode that is required for a single cell at 4Ah capacity. Atomic weight of elements in gram: Si=28 , Li=7, Mn=55, Fe=56, P=31, and O=16. If the building block cell is designed in a cylindrical format (2cm diameter and 10 cm height), calculate the energy density (Wh/lit) and specific energy (Wh/kg) at the cell level and at the battery pack level. Assume cell weight 100g, and cells are arranged in two layers in the battery pack with top…arrow_forwardProblem 2 Consider the following feedback control system. (i) (ii) K(s+2) s(s + 1)(s+3) 5+6 5+7 Use Routh-Hurwitz criterion to find the range of K for which the closed-loop system is stable. Using the Routh table from part (a), find the range of K for which the closed-loop system will have one pole in the ORHP and rest of the poles in the OLHP. This implies there will be only one sign changes in the 1st column.arrow_forward
- Problem 3 Consider the following system where x(t) denotes displacement of the mass from its equilibrium position and u(t) denotes the force applied to the mass. 28 N/m -0000-5 kg. u(t) -x(t) 5 N-s/m (a) Find the transfer function of the system. (b) Is the system internally stable (marginally or strictly) and BIBO stable? (c) Find the settling time, rise time, peak time and percent overshoot for the step-response of the system.arrow_forwardSolve this problem and show all of the workarrow_forwardSolve this problem and show all of the workarrow_forward
- 2. Determine the reactions, and shear and moment diagrams. EI= 50000 kip-ft2[50pts] Note: You can use the virtual work method/ Table to calculate fij terms. A 18 ft B 40 k 6 ft Carrow_forwardOne end of a thin uniform rod of mass m and length 31 rests against a smooth vertical wall. The other end of the rod is attached by a string of length I to a fixed point which is located a distance 21 from the wall. A horizontal force of magnitude F, is applied to the lower end of the rod as shown. Assuming the rod and the string remain in the same vertical plane perpendicular to the wall, find the angle 9 between the rod and the wall at the position of static equilibrium. Notes: This quiz is going to walk you through a sequence of steps to do this. It won't give you the answers, but it will hopefully get you to see how to approach problems like this so that you have a working reference/template in the future. This is actually a modified version of a problem from the textbook (6.3). Note that in that problem, is not actually given. It has been introduced for convenience as we move through solving the problem, and should not show up in the final answer. DO NOT DO PROBLEM 6.3. It is not…arrow_forward12.31 The voltage source in the circuit of Fig. P12.31 is, given by vs(t)= [105u(t)] V. Determine i̟L (t) for t≥0, given that R₁ =1, R2 = 12, L = 2 H, and C = 1 F. vs(t) R₁ R₂ iL L Figure P12.31 Circuit for Problems 12.31 and 12.35.arrow_forward
 MATLAB: An Introduction with ApplicationsEngineeringISBN:9781119256830Author:Amos GilatPublisher:John Wiley & Sons Inc
MATLAB: An Introduction with ApplicationsEngineeringISBN:9781119256830Author:Amos GilatPublisher:John Wiley & Sons Inc Essentials Of Materials Science And EngineeringEngineeringISBN:9781337385497Author:WRIGHT, Wendelin J.Publisher:Cengage,
Essentials Of Materials Science And EngineeringEngineeringISBN:9781337385497Author:WRIGHT, Wendelin J.Publisher:Cengage, Industrial Motor ControlEngineeringISBN:9781133691808Author:Stephen HermanPublisher:Cengage Learning
Industrial Motor ControlEngineeringISBN:9781133691808Author:Stephen HermanPublisher:Cengage Learning Basics Of Engineering EconomyEngineeringISBN:9780073376356Author:Leland Blank, Anthony TarquinPublisher:MCGRAW-HILL HIGHER EDUCATION
Basics Of Engineering EconomyEngineeringISBN:9780073376356Author:Leland Blank, Anthony TarquinPublisher:MCGRAW-HILL HIGHER EDUCATION Structural Steel Design (6th Edition)EngineeringISBN:9780134589657Author:Jack C. McCormac, Stephen F. CsernakPublisher:PEARSON
Structural Steel Design (6th Edition)EngineeringISBN:9780134589657Author:Jack C. McCormac, Stephen F. CsernakPublisher:PEARSON Fundamentals of Materials Science and Engineering...EngineeringISBN:9781119175483Author:William D. Callister Jr., David G. RethwischPublisher:WILEY
Fundamentals of Materials Science and Engineering...EngineeringISBN:9781119175483Author:William D. Callister Jr., David G. RethwischPublisher:WILEY





