
Concept explainers
RIPs as Cancer Drugs Researchers are taking a page from the structure-function relationship of RIPs in their quest for cancer treatments. The most toxic RIPs, remember, have one domain that interferes with ribosomes, and another that carries them into cells. Melissa Cheung and her colleagues incorporated a peptide that binds to skin cancer cells into the enzymatic part of an RIP, the E. coli Shiga-like toxin. The researchers created a new RIP that specifically kills .skin cancer cells, which are notoriously resistant to established therapies. Some of their results are shown in FIGURE 9.17.
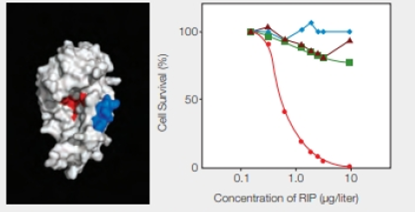
FIGURE 9.17 Effect of an engineered RIP on cancer cells. The model on the left shows the enzyme portion of E. coli Shiga-like toxin engineered to carry a small sequence of amino acids (in blue) that targets skin cancer cells. (Red indicates the active site.) The graph on the right shows the effect of this engineered RIP on human cancer cells of the skin ( ); breast (
); breast ( ) liver (
) liver ( ); and prostate (
); and prostate ( ).
).
Which cells had the greatest response to an increase in concentration of the engineered RIP?
To determine: The type of cells that had the greatest response to an increase in the concentration of the engineered RIP.
Introduction: Ribosome-inactivating proteins (RIPs) inactivate the ribosomes and prevent protein synthesis in a cell. The toxic RIPs have a domain that makes them enter into the cell and another domain that interferes with the ribosome. They have antiviral and anticancer properties and are used to design drugs for HIV and cancer.
Answer to Problem 1DAA
Correct answer: The greatest response in the form of fall in cell’s survival percentage with an increase in the concentration of engineered RIP is seen in the skin cancer cells.
Explanation of Solution
As given in the problem statement, Researcher M and her colleagues incorporated a peptide into the enzymatic part of a RIP, the E. coli Shiga-like toxin. The peptide specifically binds to the skin cancer cells, and thus, the newly synthesized RIP kills the skin cancer cells.
Refer Fig. 9.17, “Effect of an engineered RIP on cancer cells”, in the textbook. The model shown on the left indicates a blue-colored enzyme region of E. coli Shiga-like toxin that is engineered to carry the peptide sequence specific for the skin cancer cells. The red color indicates the active site of RIP.
The graphical representation that is shown in Fig. 9.17 on the right side indicates the effect of the engineered RIP on different human cancer cells indicated by different colors and shapes. They include skin, breast, liver, and prostate cancer cells with red, blue, brown, and green color, respectively. The concentration of RIP (µg/liter) is plotted with the percentage of cell survival. As shown in the graph, as the concentration of RIP increases, there is a significant drop in the skin cancer cells percentage. It reaches to zero at RIP concentration of 10 µg/liter. In the case of the other cancer cells, there is lesser variability.
Thus, the greatest response in the form of fall in cell’s survival percentage with an increase in the concentration of engineered RIP is seen in the skin cancer cells.
Want to see more full solutions like this?
Chapter 9 Solutions
Biology: The Unity and Diversity of Life
Additional Science Textbook Solutions
Fundamentals of Physics Extended
Laboratory Manual For Human Anatomy & Physiology
SEELEY'S ANATOMY+PHYSIOLOGY
Microbiology Fundamentals: A Clinical Approach
Human Physiology: An Integrated Approach (8th Edition)
- examples of synamtomorphy.arrow_forwardE. Bar Graph Use the same technique to upload the completed image. We will use a different type of graph to derive additional information from the CO2 data (Fig A1.6.2) 1. Calculate the average rate of increase in COz concentration per year for the time intervals 1959-1969, 1969- 1979, etc. and write the results in the spaces provided. The value for 1959-1969 is provided for you as an example. 2. Plot the results as a bar graph. The 1959-1969 is plotted for you. 3. Choose the graph that looks the most like yours A) E BAR GRAPH We will use a different type of graph to derive additional information from the CU, data (rig. nive). Average Yearly Rate of Observatory, Hawall interval Rate of increase per year 1959-1969 0.9 1969-1979 1979-1989 1989-1999 1999-2009 Figure A1.6.2 1999-2009 *- mrame -11- -n4 P2 جية 1989-1999 1979-1989 1969-1979 1959-1969 This bar drawn for you as an example 1.0 CO, Average Increase/Year (ppmv) B) E BAR GRAPH We will use a different type of graph to derive…arrow_forwardUse the relationships you just described to compute the values needed to fill in the blanks in the table in Fig A1.4.1 depth (a) 1.0 cml 0.7 cml cm| base dimensions (b, c)| 1.0 cm| 1.0 cm| 1.0 cm 1.0 cm| 1.0 cm| 1.0 cm volume (V) 1.0_cm' cm'| cm'| density (p) 1.0 g/cm'| 1.0 g/cm 1.0 g/cm' mass (m)| 0.3 g Column 1: depth at 1.0 cm volume mass Column 2: depth at 0.7 cm volume mass Column 3: unknown depth depth volumearrow_forward
- San Andreas Transform Boundary Plate Motion The geologic map below of southern California shows the position of the famous San Andreas Fault, a transform plate boundary between the North American Plate (east side) and the Pacific Plate (west side). The relative motion between the plates is indicated by the half arrows along the transform plate boundary (i.e., the Pacific Plate is moving to the northwest relative to the North American Plate). Note the two bodies of Oligocene volcanic rocks (labeled Ov) on the map in the previous page located along either side of the San Andreas Fault. These rocks are about 23.5 million years old and were once one body of rock. They have been separated by displacement along the fault. 21. Based on the offset of these volcanic rocks, what is the average annual rate of relative plate motion in cm/yr? SAF lab 2.jpg Group of answer choices 0.67 cm/yr 2 cm/yr 6.7 cm/yr 1.5 cm/yr CALIFORNIA Berkeley San Francisco K Os Q San Andreas Fault Ov…arrow_forwardThese are NOT part of any graded assignment. Are there other examples of synapomorphy. What is it called when the traits retained are similar to ancestors?arrow_forwardPlease hand draw everying. Thank you! Draw a gram positive bacterial cell below. Your cell should have the following parts, labeled: A coccus shape A capsule The gram positive cell wall should have the peptidoglycan labeled, as well as its component parts (NAM, NAG, and teichoic acid) A cell membrane Fimbriae A nucleoid Ribosomes Inclusionsarrow_forward
- Draw a gram negative bacterial cell below. Your cell should have the following parts, labeled: A bacillus shape Fimbriae Amphitrichous flagella 2 membranes (outer and inner) The outer membrane should have lipopolysaccharide (LPS) with lipid A and O antigens Periplasmic space The thin peptidoglycan cell wall between the 2 membranes A nucleoid Ribosomes Inclusionsarrow_forwardBacterial species Cell wall type Example: S. mitis Gram positive S. epidermidis H. pylori M. bovis S. marcescens Shape and arrangement Coccus, streptococcus Drawing 0000000arrow_forwardDraw a gram positive bacterial cell below. Your cell should have the following parts, labeled: A coccus shape A capsule The gram positive cell wall should have the peptidoglycan labeled, as well as its component parts (NAM, NAG, and teichoic acid) A cell membrane Fimbriae A nucleoid Ribosomes Inclusionsarrow_forward
 Biology: The Unity and Diversity of Life (MindTap...BiologyISBN:9781305073951Author:Cecie Starr, Ralph Taggart, Christine Evers, Lisa StarrPublisher:Cengage Learning
Biology: The Unity and Diversity of Life (MindTap...BiologyISBN:9781305073951Author:Cecie Starr, Ralph Taggart, Christine Evers, Lisa StarrPublisher:Cengage Learning Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning
Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning
Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning
Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning





