
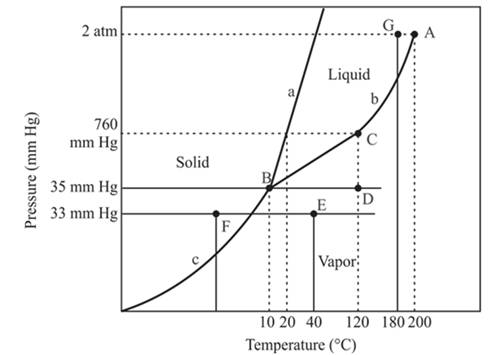
Consider the phase diagram of the compound in Problem 17 to answer the following questions.
(a) What is the physical state of the compound at 35 mm Hg and 120°C?
(b) What is the normal freezing point of the compound?
(c) What is the point A called?
(d) What is the point B called?
(e) What is the point C called?
(f) What change occurs when at a constant pressure of 33 mm Hg, the temperature is decreased from 40°C to -20°C?
(g) Will the solid float on the liquid?
(h) Can the compound exist as a liquid at 180°C and 2 atm pressure?
(a)
Interpretation:
Refer to the given phase diagram of compound X. The physical state of compound X is to be determined at pressure 35mmHg and temperature 120°C.
Concept introduction:
Phase diagram can be defined as the representation of pressure and temperature quantities of any pure substance. At these values of temperature and pressure, the different phases of the pure substance are equilibrium to each other.
Answer to Problem 18QAP
The physical state of compound X is vapor determined at pressure 35mmHg and temperature 120°C.
Explanation of Solution
Given Infromation:
The phase diagram of the compound X is as follows:
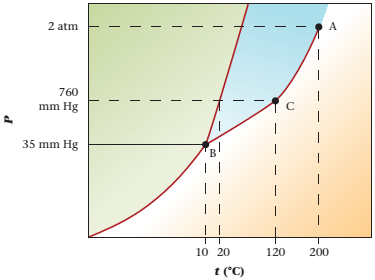
The triple point is defined as the equilibrium condition among all the three phases of any pure substance which is solid, liquid and gas. The curves are used for the equilibrium condition between two phases of pure substance. The phase diagram is shown below-
In the below diagram −
a = solid-liquid phase in equilibrium
b = liquid-vapor phase in equilibrium
c = solid-vapor phase in equilibrium
Given values-
Pressure = 35mmHg
Temperature = -50°C
These two given values are intersecting at point D and point D lies in the vapor phase region.
Hence,
At pressure 35 mmHg and at temperature 120°C, the physical state of the compound is vapor.
(b)
Interpretation:
The normal freezing point of a compound is to be determined.
Concept introduction:
Phase diagram can be defined as the representation of pressure and temperature quantities of any pure substance. At these values of temperature and pressure, the different phases of the pure substance are equilibrium to each other.
Answer to Problem 18QAP
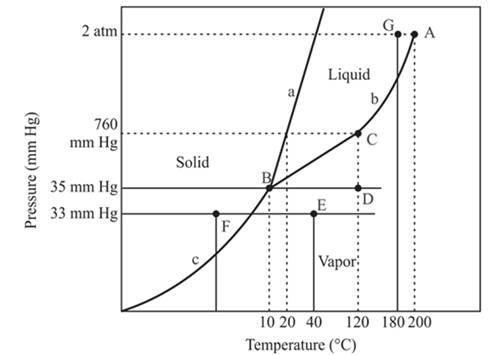
The normal freezing point of a compound is 20°C.
Explanation of Solution
The triple point is defined as the equilibrium condition among all the three phases of any pure substance which is solid, liquid and gas. The curves are used for the equilibrium condition between two phases of pure substance. The phase diagram is shown below-
In the above diagram −
a = solid-liquid phase in equilibrium
b = liquid-vapor phase in equilibrium
c = solid-vapor phase in equilibrium
The normal boiling temperature is the temperature at which compound start freezing which is basically atmospheric pressure. Therefore pressure for the freezing point is 760mmHg. At atmospheric pressure of freezing point, if at the particular temperature the liquid and vapor phase of a substance are at equilibrium then that temperature is the freezing point temperature. From the phase diagram of the compound, at temperature 20°C liquid and vapor phase of a substance is at the equilibrium.
Hence, the freezing point temperature is 20°C.
(c)
Interpretation:
The name for the point A is to be determined.
Concept introduction:
Phase diagram can be defined as the representation of pressure and temperature quantities of any pure substance. At these values of temperature and pressure, the different phases of the pure substance are equilibrium to each other.
Answer to Problem 18QAP
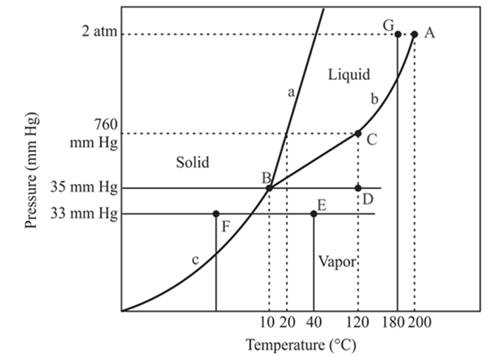
The A is the critical point of the phase diagram of the compound.
Explanation of Solution
The triple point is defined as the equilibrium condition among all the three phases of any pure substance which is solid, liquid and gas. The curves are used for the equilibrium condition between two phases of water. The phase diagram is shown below-
In the above diagram −
a = solid-liquid phase in equilibrium
b = liquid-vapor phase in equilibrium
c = solid-vapor phase in equilibrium
From the above diagram, the critical point of a substance is represented by the end of that curve which shows the equilibrium condition between the liquid phase and vapor phase. This curve is curved b and it ends at point A at temperature 200°C and at pressure 2 atm.
Hence, the point A is called a critical point.
(d)
Interpretation:
The name for the point B is to be determined.
Concept introduction:
Phase diagram can be defined as the representation of pressure and temperature quantities of any pure substance. At these values of temperature and pressure, the different phases of the pure substance are equilibrium to each other.
Answer to Problem 18QAP
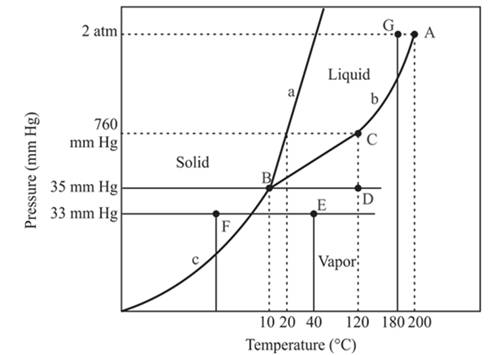
The point B is the triple point in the phase diagram.
Explanation of Solution
The triple point is defined as the equilibrium condition among all the three phases of any pure substance which is solid, liquid and gas. The curves are used for the equilibrium condition between two phases of water. The phase diagram is shown below-
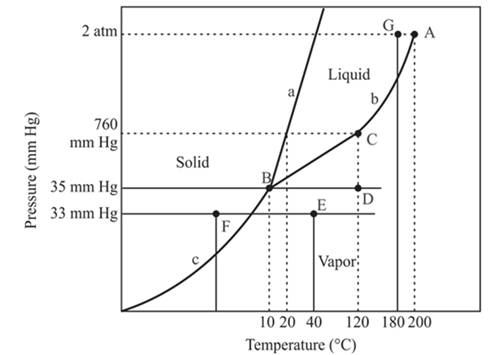
In the above diagram −
a = solid-liquid phase in equilibrium
b = liquid-vapor phase in equilibrium
c = solid-vapor phase in equilibrium
The triple point represents the equilibrium among all the phases which are solid, liquid and gas of compound. Hence, the intersection point of all curves am and c will represent the triple point. From the graph, the intersection point of these three curves is existing at point B.
Therefore, point B will represent the triple point of the compound.
(e)
Interpretation:
The name for the point C is to be determined.
Concept introduction:
Phase diagram can be defined as the representation of pressure and temperature quantities of any pure substance. At these values of temperature and pressure, the different phases of the pure substance are equilibrium to each other.
Answer to Problem 18QAP
The point C is the normal boiling point for the compound.
Explanation of Solution
The triple point is defined as the equilibrium condition among all the three phases of any pure substance which is solid, liquid and gas. The curves are used for the equilibrium condition between two phases of water. The phase diagram is shown below-
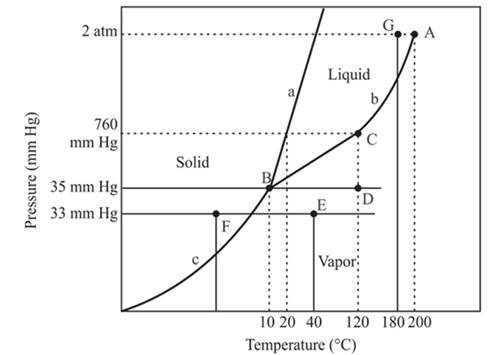
In the above diagram −
a = solid-liquid phase in equilibrium
b = liquid-vapor phase in equilibrium
c = solid-vapor phase in equilibrium
The point C is the normal boiling point at which the compound starts boiling and the pressure for this is atmospheric pressure or 1 atm or 760 mmHg.
(f)
Interpretation:
The changes are to be determined at constant pressure 33mmHg and the temperature is changing from 40°C to -20°C.
Concept introduction:
Phase diagram can be defined as the representation of pressure and temperature quantities of any pure substance. At these values of temperature and pressure, the different phases of the pure substance are equilibrium to each other.
Answer to Problem 18QAP
At constant pressure 33mmHg and the process of changing of temperature from 40°C to -20°C is occurred between point E and F. In this process-
There is a change of phase.
Explanation of Solution
The triple point is defined as the equilibrium condition among all the three phases of any pure substance which is solid, liquid and gas. The curves are used for the equilibrium condition between two phases of water. The phase diagram is shown below-
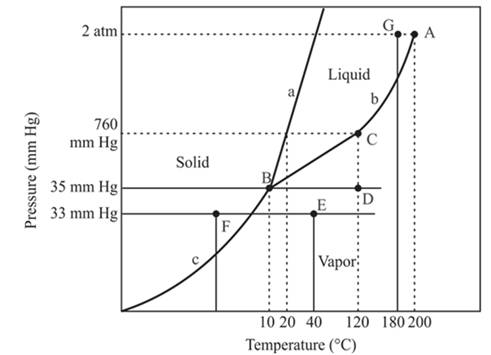
In the above diagram −
a = solid-liquid phase in equilibrium
b = liquid-vapor phase in equilibrium
c = solid-vapor phase in equilibrium
In this process the, the pressure is constant and the temperature varies from 40°C to -20°C which is represented by point E and F. The starting point is E and endpoint is F and both have different phases. The point E or starting point occurs in the vapor phase and the ending point, point F lies in the solid phase. So, there is an occurrence of a change of phase.
(g)
Interpretation:
The statement that solid float on liquid is to be checked.
Concept introduction:
Phase diagram can be defined as the representation of pressure and temperature quantities of any pure substance. At these values of temperature and pressure, the different phases of the pure substance are equilibrium to each other.
Answer to Problem 18QAP
The densest phase is a solid phase.
Explanation of Solution
The triple point is defined as the equilibrium condition among all the three phases of any pure substance which is solid, liquid and gas. The curves are used for the equilibrium condition between two phases of water. The phase diagram is shown below-
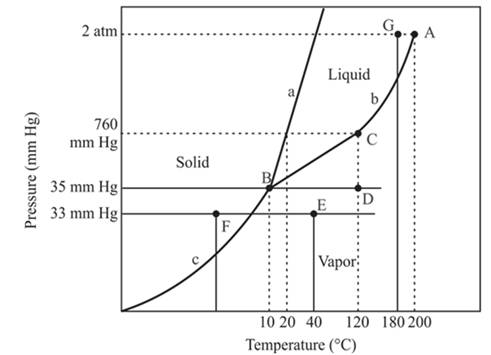
In the above diagram −
a = solid-liquid phase in equilibrium
b = liquid-vapor phase in equilibrium
c = solid-vapor phase in equilibrium
The floating of solid on liquid depends on the density of these two phases. In the case of the solid phase of water, it will float on the liquid phase of water. But this condition is not the same in all condition because the solid iron piece will not be able to float on the liquid water otherwise it will sink in the water.
(h)
Interpretation:
The existence of compound at pressure 2 atm and at temperature 180°C is to be determined.
Concept introduction:
Phase diagram can be defined as the representation of pressure and temperature quantities of any pure substance. At these values of temperature and pressure, the different phases of the pure substance are equilibrium to each other.
Answer to Problem 18QAP
The compound at pressure 2 atm and at temperature 180°C will exist as a liquid.
Explanation of Solution
The triple point is defined as the equilibrium condition among all the three phases of any pure substance which is solid, liquid and gas. The curves are used for the equilibrium condition between two phases of water. The phase diagram is shown below-
In the above diagram −
a = solid-liquid phase in equilibrium
b = liquid-vapor phase in equilibrium
c = solid-vapor phase in equilibrium
The compound with pressure 2 atm and at temperature 180°C is occurred at point G which exist at the left of the critical point or point A. The pressure of this pint is less than the critical point hence it will exist in the critical limits. Therefore, the phase of the compound at this point is the liquid phase. Therefore, compound will exist as a liquid at given quantities.
Want to see more full solutions like this?
Chapter 9 Solutions
EBK CHEMISTRY: PRINCIPLES AND REACTIONS
- When 15.00 mL of 3.00 M NaOH was mixed in a calorimeter with 12.80 mL of 3.00 M HCl, both initially at room temperature (22.00 C), the temperature increased to 29.30 C. The resultant salt solution had a mass of 27.80 g and a specific heat capacity of 3.74 J/Kg. What is heat capacity of the calorimeter (in J/C)? Note: The molar enthalpy of neutralization per mole of HCl is -55.84 kJ/mol.arrow_forwardQ6: Using acetic acid as the acid, write the balanced chemical equation for the protonation of the two bases shown (on the -NH2). Include curved arrows to show the mechanism. O₂N- O₂N. -NH2 -NH2 a) Which of the two Bronsted bases above is the stronger base? Why? b) Identify the conjugate acids and conjugate bases for the reactants. c) Identify the Lewis acids and bases in the reactions.arrow_forwardQ5: For the two reactions below: a) Use curved electron-pushing arrows to show the mechanism for the reaction in the forward direction. Redraw the compounds to explicitly illustrate all bonds that are broken and all bonds that are formed. b) Label Bronsted acids and bases in the left side of the reactions. c) For reaction A, which anionic species is the weakest base? Which neutral compound is the stronger acid? Is the forward or reverse reaction favored? d) Label Lewis acids and bases, nucleophiles and electrophiles in the left side of the reactions. A. 용 CH3OH я хон CH3O OH B. HBr CH3ONa NaBr CH3OHarrow_forward
- potential energy Br b) Translate the Newman projection below to its wedge-and-dash drawing. F H. OH CH3 CI c) Isopentane (2-methylbutane) is a compound containing a branched carbon chain. Draw a Newman projection of six conformations about the C2-C3 bond of isopentane. On the curve of potential energy versus angle of internal rotation for isopentane, label each energy maximum and minimum with one of the conformations. 0° 。 F A B D C angle of internal rotation E F 360° (=0°) JDownlarrow_forwardQ7: Identify the functional groups in these molecules a) CH 3 b) Aspirin: HO 'N' Capsaicin HO O CH3 CH 3arrow_forwardQ2: Name the following alkanesarrow_forward
- 1. Complete the following table in your laboratory notebook. Substance Formula Methanol CH3OH Ethanol C2H5OH 1-Propanol C3H7OH 1-Butanol C4H9OH Pentane C5H12 Hexane C6H14 Water H₂O Acetone C3H60 Structural Formula Molecular Weight (g/mol) Hydrogen Bond (Yes or No)arrow_forwardQ1: Compare the relative acidity in each pair of compounds. Briefly explain. (a) CH3OH vs NH 3 (b) HF vs CH3COOH (c) NH3 vs CH4 (d) HCI vs HI (e) CH3COOH vs CH3SH (f) H₂C=CH2 vs CH3 CH3 (g) compare the acidity of the two bolded hydrogens O. H N- (h) compare the acidity of the two bolded hydrogens, draw resonance structures to explain H H Harrow_forwardQ3: Rank the following molecules in order of decreasing boiling point: (a) 3-methylheptane; (b) octane; (c) 2,4-dimethylhexane; (d) 2,2,4-trimethylpentane.arrow_forward
- Q5: Conformations of Alkanes a) Draw a Newman Projection of the compound below about the C2-C3 bond. H3C Cli... H IIIH Br CH3arrow_forwardThe ability of atoms to associate with each other depends ona) the electronic structure and its spatial orientation.b) the electron affinity.c) The other two answers are correct.arrow_forwardWhat is the final volume after you reach the final temperature? I put 1.73 but the answer is wrong not sure why The initial volume of gas is 1.60 LL , the initial temperature of the gas is 23.0 °C°C , and the system is in equilibrium with an external pressure of 1.2 bar (given by the sum of a 1 bar atmospheric pressure and a 0.2 bar pressure due to a brick that rests on top of the piston). Then, as you did in Exercise 1, you heat the gas slowly until the temperature reaches 48.2 °Carrow_forward
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning





