
(a)
Interpretation:
Refer to the given phase diagram of compound X. The physical state of compound X is to be determined at pressure 35mmHg and temperature -50°C.
Concept introduction:
Phase diagram can be defined as the representation of pressure and temperature quantities of any pure substance. At these values of temperature and pressure, the different phases of the pure substance are equilibrium to each other.
Answer to Problem 17QAP
The physical state of compound X is solid determined at pressure 35mmHg and temperature -50°C.
Explanation of Solution
Given Information:
The phase diagram of compound X is as follows:
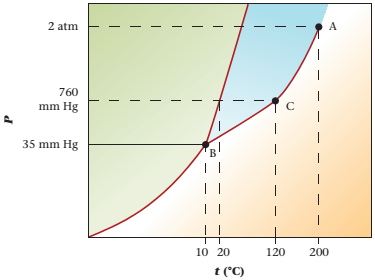
The triple point is defined as the equilibrium condition among all the three phases of any pure substance which is solid, liquid and gas. The curves are used for the equilibrium condition between two phases of pure substance. The phase diagram is shown below-
In the below diagram −
a = solid-liquid phase in equilibrium
b = liquid-vapor phase in equilibrium
c = solid-vapor phase in equilibrium
Given values-
Pressure = 35mmHg
Temperature = -50°C
These two given values are represented in the plot of the phase diagram of compound X. These two quantities are existing at the left of point B.
Hence,
At pressure 35 mmHg and at temperature -50°C, the physical state of the compound is solid.
(b)
Interpretation:
The normal boiling point of a compound is to be determined.
Concept introduction:
Phase diagram can be defined as the representation of pressure and temperature quantities of any pure substance. At these values of temperature and pressure, the different phases of the pure substance are equilibrium to each other.
Answer to Problem 17QAP
The normal boiling point of a compound is 120°C.
Explanation of Solution
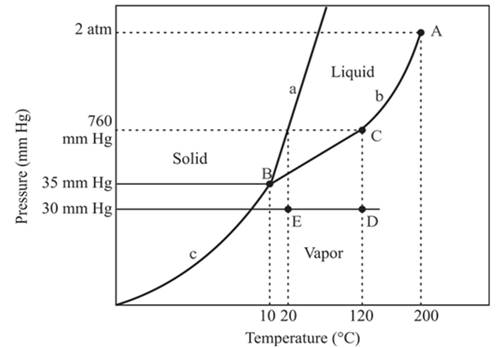
The triple point is defined as the equilibrium condition among all the three phases of any pure substance which is solid, liquid and gas. The curves are used for the equilibrium condition between two phases of pure substance. The phase diagram is shown below-
In the above diagram −
a = solid-liquid phase in equilibrium
b = liquid-vapor phase in equilibrium
c = solid-vapor phase in equilibrium
The normal boiling temperature is the temperature at which compound start boiling which is basically atmospheric pressure. Therefore pressure for the boiling point is 760mmHg. At atmospheric pressure of boiling point, if at the particular temperature the liquid and vapor phase of a substance are at equilibrium, then that temperature is the boiling point temperature. From the phase diagram of substance at temperature, 120°C liquid and vapor phase of a substance is at the equilibrium.
Hence, the boiling point temperature is 120°C.
(c)
Interpretation:
The critical point for the compound is to be located.
Concept introduction:
Phase diagram can be defined as the representation of pressure and temperature quantities of any pure substance. At these values of temperature and pressure, the different phases of the pure substance are equilibrium to each other.
Answer to Problem 17QAP
The critical point for the compound is located at point A.
Explanation of Solution
The triple point is defined as the equilibrium condition among all the three phases of any pure substance which is solid, liquid and gas. The curves are used for the equilibrium condition between two phases of water. The phase diagram is shown below-
In the above diagram −
a = solid-liquid phase in equilibrium
b = liquid-vapor phase in equilibrium
c = solid-vapor phase in equilibrium
From the above diagram, the critical point of a substance is represented by the end of that curve which shows the equilibrium condition between the liquid phase and vapor phase. This curve is curved b and it ends at point A at temperature 200°C and at pressure 2 atm.
Hence, the critical point will exist at point A.
(d)
Interpretation:
The triple point for the compound is to be located.
Concept introduction:
Phase diagram can be defined as the representation of pressure and temperature quantities of any pure substance. At these values of temperature and pressure, the different phases of the pure substance are equilibrium to each other.
Answer to Problem 17QAP
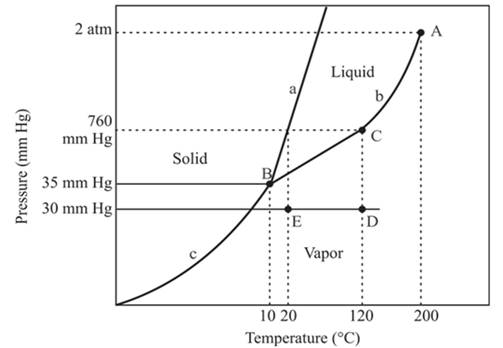
The triple point for the compound is located at point B
Explanation of Solution
The triple point is defined as the equilibrium condition among all the three phases of any pure substance which is solid, liquid and gas. The curves are used for the equilibrium condition between two phases of water. The phase diagram is shown below-
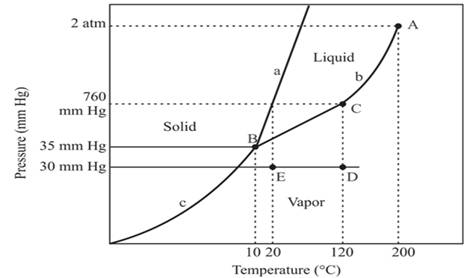
In the above diagram −
a = solid-liquid phase in equilibrium
b = liquid-vapor phase in equilibrium
c = solid-vapor phase in equilibrium
The triple point represents the equilibrium among all the phases which are solid, liquid and gas of compound. Hence, the intersection point of all curves a, b and c will represent the triple point. From the graph, the intersection point of these three curves is existing at point B.
Therefore, point B will represent the triple point of the compound.
(e)
Interpretation:
The changes are to be determined at constant pressure 30mmHg and the temperature is changing from 120°C to 20°C.
Concept introduction:
Phase diagram can be defined as the representation of pressure and temperature quantities of any pure substance. At these values of temperature and pressure, the different phases of the pure substance are equilibrium to each other.
Answer to Problem 17QAP
At constant pressure, 30mmHg and the process of changing of temperature from 120°C to 20°C is occurring between point D and E. In this process-
No change in phase.
The density of the vapor phase is high at point E in comparison to point D.
Explanation of Solution
The triple point is defined as the equilibrium condition among all the three phases of any pure substance which is solid, liquid and gas. The curves are used for the equilibrium condition between two phases of water. The phase diagram is shown below-
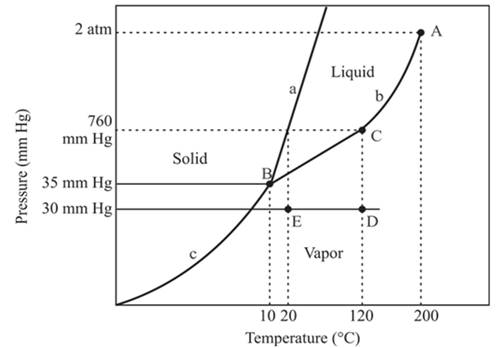
In the above diagram −
a = solid-liquid phase in equilibrium
b = liquid-vapor phase in equilibrium
c = solid-vapor phase in equilibrium
In this process the, the pressure is constant and the temperature varies from 120°C to 20°C which is represented by point D and E. the starting point D and end point E both are existing in the vapor phase. It means there is no phase change when the temperature is decreasing form 120°C to 20°C. The density of the vapor phase at point E will be increased because it is nearer to the liquid and the solid phase in comparison to point D.
(f)
Interpretation:
The densest phase is to be determined.
Concept introduction:
Phase diagram can be defined as the representation of pressure and temperature quantities of any pure substance. At these values of temperature and pressure, the different phases of the pure substance are equilibrium to each other.
Answer to Problem 17QAP
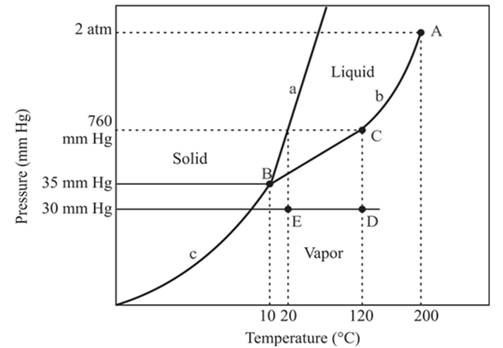
The densest phase is a solid phase.
Explanation of Solution
The triple point is defined as the equilibrium condition among all the three phases of any pure substance which is solid, liquid and gas. The curves are used for the equilibrium condition between two phases of water. The phase diagram is shown below-
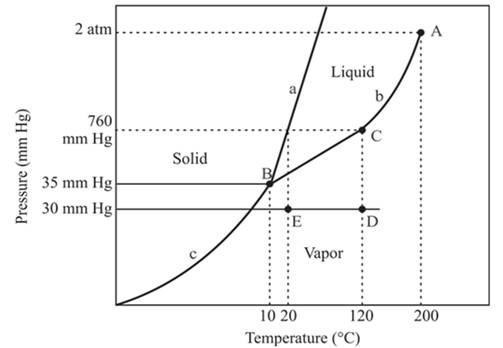
In the above diagram −
a = solid-liquid phase in equilibrium
b = liquid-vapor phase in equilibrium
c = solid-vapor phase in equilibrium
The densest part of this phase diagram is a solid phase because in solid phase all molecules are closely bound and from the diagram, we can see that the solid phase exists at the high temperature and low pressure, therefore, solid phase is the densest phase among all.
Want to see more full solutions like this?
Chapter 9 Solutions
Bundle: Chemistry: Principles and Reactions, 8th, Loose-Leaf + OWLv2, 1 term (6 months) Printed Access Card
- Part 3: AHm,system Mass of 1.00 M HCI Vol. of 1.00 M HCI Mass of NaOH(s) Total Mass in Calorimeter Mole product if HCI limiting reactant Trial 1 62.4009 1.511g Mole product if NaOH limiting reactant Limiting reactant Initial Temperature Final Temperature 23.8°C 37.6°C Change in Temperature AHm,system (calculated) Average AHm,system (calculated) (calculated) (calculated) Trial 2 64.006g 1.9599 (calculated) (calculated) (calculated) (calculated) (calculated) (calculated) 24.7°C 41.9°C (calculated) (calculated) (2 pts. each)arrow_forwardDon't used Ai solutionarrow_forwardWhat is the numerical value of the slope using the equation y=-1.823x -0.0162 please show calculationsarrow_forward
- Don't used hand raitingarrow_forward1.) Using the graph below (including the line equation of y = -1.823x - 0.0162) What is the numerical value for the slope shown? 2.) What are the Unit(s) associated with the slope of the line shown? for we all remember that numerical data always has units. 3.) What would be a good title for this graph and explain your choice. 0.00 0.0 02 0.4 10.6 08 10 12 -0.20 -0.40 -0.60 -0.80 Temp, freezing, in degrees Celcius 5-1.00 -1.20 -1.40 -1:60 y=-1.823x-0.0162 -180 -2.00 Concentration of Sucrose (m)arrow_forwardDon't used Ai solutionarrow_forward
- Identify the Functional Groups (FG) in the following molecules. Classify C atoms as tertiary, 30, or quaternary 40. Identify secondary 20 and tertiary, 30 hydrogen atoms. Please provide steps to undertand each labeling. Please label in the image, so it fits explanation. I am still very unsure I undertand this.arrow_forwardDon't used Ai solutionarrow_forwardDon't used Ai solutionarrow_forward
- 3. Devise a retrosynthesis for the problem given below and then provide the corresponding synthesis with all necessary reagents/reactants: RETROSYNTHESIS: SYNTHESIS: Brarrow_forwardSeveral square planar complexes are known for Gold (III) ions but not for Silver (III) why?arrow_forwardAiter running various experiments, you determine that the mechanism for the following reaction is bimolecular. CI Using this information, draw the correct mechanism in the space below. X Explanation Check C Cl OH + CI Add/Remove step Click and drag to start drawing a structure. 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Carrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning





