
Concept explainers
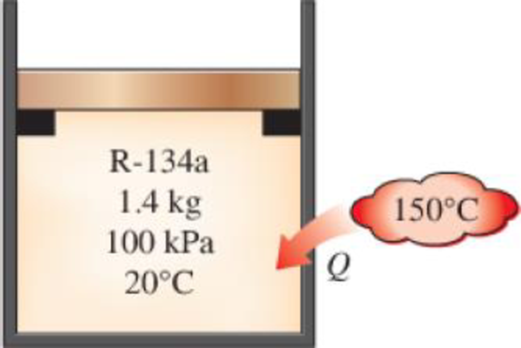
A piston–cylinder device initially contains 1.4 kg of refrigerant-134a at 100 kPa and 20°C. Heat is now transferred to the refrigerant from a source at 150°C, and the piston, which is resting on a set of stops, starts moving when the pressure inside reaches 120 kPa. Heat transfer continues until the temperature reaches 80°C. Assuming the surroundings to be at 25°C and 100 kPa, determine (a) the work done, (b) the heat transfer, (c) the exergy destroyed, and (d) the second-law efficiency of this process.
FIGURE P8–48
(a)
The work done.
Answer to Problem 48P
The work done is
Explanation of Solution
Express the boundary work done.
Here, mass is
Conclusion:
Perform the unit conversion of initial pressure and final pressure from
Refer Table A-13, “superheated refrigerant-134a”, and write the properties corresponding to initial pressure of
Here, initial specific volume, internal energy and entropy is
Refer Table A-13, “superheated refrigerant-134a”, and write the properties corresponding to final pressure of
Write the formula of interpolation method of two variables.
Here, the variables denote by x and y is final pressure and final specific volume respectively.
Show the final specific volume at
|
Final pressure |
Final specific volume |
| 0.10 | 0.28465 |
| 0.12 | |
| 0.14 | 0.20242 |
Substitute
Show the final specific internal energy at
|
Final pressure |
Final specific internal energy |
| 0.10 | 297.10 |
| 0.12 | |
| 0.14 | 296.77 |
Substitute
Show the final specific entropy at
|
Final pressure |
Final specific entropy |
| 0.10 | 1.2573 |
| 0.12 | |
| 0.14 | 1.2289 |
Substitute
Thus, write the values obtained from interpolation method:
Substitute
Hence, the work done is
(b)
The heat transfer.
Answer to Problem 48P
The heat transfer is
Explanation of Solution
Express heat transfer.
Conclusion:
Substitute 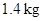 for
for  ,
,
(c)
The exergy destroyed.
Answer to Problem 48P
The exergy destroyed is
Explanation of Solution
Express the exergy destruction.
Here, entropy generation is
Express the entropy generation by taking entropy balance on an extended system.
Here, net entropy transfer by heat and mass is
Conclusion:
Substitute
Substitute
Hence, the exergy destroyed is
(d)
The second law efficiency of the process.
Answer to Problem 48P
The second law efficiency of the process is
Explanation of Solution
Express exergy expended.
Here, efficiency for reversible cycle is
Express the second law efficiency.
Conclusion:
Substitute
Substitute
Hence, the second law efficiency of the process is
Want to see more full solutions like this?
Chapter 8 Solutions
EBK THERMODYNAMICS: AN ENGINEERING APPR
- A mass of ideal gas in a closed piston-cylinder system expands from 427 °C and 16 bar following the process law, pv1.36 = Constant (p times v to the power of 1.36 equals to a constant). For the gas, initial : final pressure ratio is 4:1 and the initial gas volume is 0.14 m³. The specific heat of the gas at constant pressure, Cp = 0.987 kJ/kg-K and the specific gas constant, R = 0.267 kJ/kg.K. Determine the change in total internal energy in the gas during the expansion. Enter your numerical answer in the answer box below in KILO JOULES (not in Joules) but do not enter the units. (There is no expected number of decimal points or significant figures).arrow_forwardmy ID# 016948724. Please solve this problem step by steparrow_forwardMy ID# 016948724 please find the forces for Fx=0: fy=0: fz=0: please help me to solve this problem step by steparrow_forward
- My ID# 016948724 please solve the proble step by step find the forces fx=o: fy=0; fz=0; and find shear moment and the bending moment diagran please draw the diagram for the shear and bending momentarrow_forwardMy ID#016948724. Please help me to find the moment of inertia lx ly are a please show to solve step by stepsarrow_forwardplease solve this problem step by steparrow_forward
- Please do not use any AI tools to solve this question. I need a fully manual, step-by-step solution with clear explanations, as if it were done by a human tutor. No AI-generated responses, please.arrow_forwardPlease do not use any AI tools to solve this question. I need a fully manual, step-by-step solution with clear explanations, as if it were done by a human tutor. No AI-generated responses, please.arrow_forwardPlease do not use any AI tools to solve this question. I need a fully manual, step-by-step solution with clear explanations, as if it were done by a human tutor. No AI-generated responses, please.arrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





