
CHEMISTRY THE CENTRAL SCIENCE 14TH EDI
14th Edition
ISBN: 9780134863016
Author: Brown
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 8.7, Problem 8.11.2PE
(a)
Interpretation Introduction
To determine: The atom that is never found with more than an octet of valence electrons out of the given elements 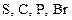 and
and 
(b)
Interpretation Introduction
To determine: The Lewis structure of 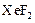
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Explain reasons as to why the amount of caffeine extracted from both a singular extraction (5ml Mountain Dew) and a multiple extraction (2 x 5.0ml Mountain Dew) were severely high when compared to coca-cola?
Protecting Groups and Carbonyls
6) The synthesis generates allethrolone that exhibits high insect toxicity but low mammalian toxicity. They are used in pet
shampoo, human lice shampoo, and industrial sprays for insects and mosquitos. Propose detailed mechanistic steps to
generate the allethrolone label the different types of reagents (Grignard, acid/base protonation, acid/base deprotonation,
reduction, oxidation, witting, aldol condensation, Robinson annulation, etc.)
III + VI
HS
HS
H+
CH,CH,Li
III
I
II
IV
CI + P(Ph)3
V
༼
Hint: no strong base added
VI
S
VII
IX
HO
VIII
-MgBr
HgCl2,HgO
HO.
isomerization
aqeuous solution
H,SO,
༽༽༤༽༽
X
MeOH
Hint: enhances selectivity for reaction at the S
X
☑
Draw the complete mechanism for the acid-catalyzed hydration of this alkene.
esc
田
Explanation
Check
1
888
Q
A
slock
Add/Remove step
Q
F4
F5
F6
A
བྲA
F7
$
%
5
@
4
2
3
&
6
87
Click and drag to start
drawing a structure.
© 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Ce
W
E
R
T
Y
U
S
D
LL
G
H
IK
DD
요
F8
F9
F10
F1
*
(
8
9
0
O
P
J
K
L
Z
X
C
V
B
N
M
H
He
command
Chapter 8 Solutions
CHEMISTRY THE CENTRAL SCIENCE 14TH EDI
Ch. 8.2 - Which of the these elements is most likely to from...Ch. 8.2 - Prob. 8.1.2PECh. 8.2 - Which of the following bond is the most polar? H-F...Ch. 8.2 - Prob. 8.2.2PECh. 8.3 - Prob. 8.3.1PECh. 8.3 - Prob. 8.3.2PECh. 8.4 - Which of the following bonds is the most polar? a....Ch. 8.4 - Which of the following bonds is most polar: S-Cl,...Ch. 8.4 - Prob. 8.5.1PECh. 8.4 - The dipole moment of chlorine monofluoride,...
Ch. 8.5 - Which of the these molecules has a Lewis structure...Ch. 8.5 -
How many valence electrons should appear in the...Ch. 8.5 - Compare the lewis symbol for neon the structure...Ch. 8.5 - Prob. 8.7.2PECh. 8.5 - Prob. 8.8.1PECh. 8.5 - Prob. 8.8.2PECh. 8.5 - Prob. 8.9.1PECh. 8.5 - Prob. 8.9.2PECh. 8.6 - Which of the statements about resonance is true?...Ch. 8.6 - Prob. 8.10.2PECh. 8.7 - Prob. 8.11.1PECh. 8.7 - Prob. 8.11.2PECh. 8 - Prob. 1DECh. 8 - Prob. 1ECh. 8 - Prob. 2ECh. 8 - A portion of a two-dimensional "slab" of NaCl(s)...Ch. 8 - Prob. 4ECh. 8 - Prob. 5ECh. 8 - Incomplete Lewis structures for the nitrous acid...Ch. 8 - Prob. 7ECh. 8 - Prob. 8ECh. 8 - Prob. 9ECh. 8 - True or false: The hydrogen atom is most stable...Ch. 8 - Consider the element silicon, Si. Write its...Ch. 8 - Write the electron configuration for the element...Ch. 8 - Prob. 13ECh. 8 - What is the Lewis symbol for each of the following...Ch. 8 - Using Lewis symbols, diagram the reaction between...Ch. 8 - Use Lewis symbols to represent the reaction that...Ch. 8 - Predict the chemical formula of the ionic compound...Ch. 8 - Prob. 18ECh. 8 - Prob. 19ECh. 8 - Prob. 20ECh. 8 - Is lattice energy usually endothermic or...Ch. 8 - NaCI and KF have the same crystal structure. The...Ch. 8 - Prob. 23ECh. 8 - Prob. 24ECh. 8 - Consider the ionic compounds KF, NaCl, NaBr, and...Ch. 8 - Which of the following trends in lattice energy is...Ch. 8 - Energy is required to remove two electrons from Ca...Ch. 8 - Prob. 28ECh. 8 - Use data from Appendix C, Figure 7.10, and Figure...Ch. 8 - Prob. 30ECh. 8 - Prob. 31ECh. 8 - Prob. 32ECh. 8 - Using Lewis symbols and Lewis structures, diagram...Ch. 8 - Use Lewis symbols and Lewis structures to diagram...Ch. 8 - Prob. 35ECh. 8 - Prob. 36ECh. 8 - Prob. 37ECh. 8 - What is the trend in electronegativity going from...Ch. 8 - Prob. 39ECh. 8 - By referring only to the periodic table, select...Ch. 8 - which of the following bonds are polar? B-F,...Ch. 8 - Arrange the bonds in each of the following sets in...Ch. 8 - Prob. 43ECh. 8 - Prob. 44ECh. 8 - In the following pairs of binary compounds,...Ch. 8 - Prob. 46ECh. 8 - Prob. 47ECh. 8 - Write Lewis structures for the following: H2CO...Ch. 8 - Prob. 49ECh. 8 - Draw the dominant Lewis structure for the...Ch. 8 - Write Lewis structures that obey the octet rule...Ch. 8 - Prob. 52ECh. 8 - Prob. 53ECh. 8 - Prob. 54ECh. 8 - Prob. 55ECh. 8 - Prob. 56ECh. 8 - Prob. 57ECh. 8 - Prob. 58ECh. 8 - Prob. 59ECh. 8 - Prob. 60ECh. 8 - Prob. 61ECh. 8 - 8.62 For Group 3A-7A elements in the third row of...Ch. 8 - Draw the Lewis structures for each of the...Ch. 8 - Prob. 64ECh. 8 - In the vapor phase, BeCl2exists as a discrete...Ch. 8 -
8.66
Describe the molecule xenon trioxide, XeO3,...Ch. 8 -
8.67 There are many Lewis structures you could...Ch. 8 - Prob. 68ECh. 8 - Using Table 8.3, estimate H for each of the...Ch. 8 - Using Table 8.3, estimate H for the following...Ch. 8 - State whether each of these statements is true or...Ch. 8 - Prob. 72ECh. 8 - Prob. 73ECh. 8 - Prob. 74ECh. 8 - Prob. 75ECh. 8 - Prob. 76ECh. 8 - A new compound is made that has a C-C bond length...Ch. 8 - A new compound is made that has an N-N bond length...Ch. 8 - Prob. 79AECh. 8 - Prob. 80AECh. 8 - An ionic substance of formula MX has a lattice...Ch. 8 - Prob. 82AECh. 8 - Prob. 83AECh. 8 - Prob. 84AECh. 8 - Consider the collection of nonmetallic elements 0,...Ch. 8 - The substance chlorine monoxide, CIO(g), is...Ch. 8 -
[8.87]
a. using the electronegativities of Br...Ch. 8 - Prob. 88AECh. 8 - Although I3- is a known ion, F3- is not. a. Draw...Ch. 8 - Calculate the formal charge on the indicated atom...Ch. 8 - The hypochlorite ion, CIO- , is the active...Ch. 8 - Prob. 92AECh. 8 - a. Triazine, C3 H3N3, is like benzene except that...Ch. 8 - Prob. 94IECh. 8 - Prob. 95IECh. 8 - Prob. 96IECh. 8 - Prob. 97IECh. 8 - Prob. 98IECh. 8 - Prob. 99IECh. 8 - Prob. 100IECh. 8 - Prob. 101IECh. 8 - Prob. 102IECh. 8 -
8.103 The compound chloral hydrate, known in...Ch. 8 - Barium azide is 62.04% Ba and 37.96% N. Each azide...Ch. 8 - Acetylene (C2H2) and nitrogen (N2) both contain a...Ch. 8 - Prob. 106IECh. 8 - Prob. 107IECh. 8 -
8.108 Formic acid has the chemical formula...Ch. 8 - Prob. 109IECh. 8 - Prob. 110IE
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Explanation Check F1 H₂O H₂ Pd 1) MCPBA 2) H3O+ 1) Hg(OAc)2, H₂O 2) NaBH4 OH CI OH OH OH hydration halohydrin formation addition halogenation hydrogenation inhalation hydrogenation hydration ☐ halohydrin formation addition halogenation formation chelation hydrogenation halohydrin formation substitution hydration halogenation addition Ohalohydrin formation subtraction halogenation addition hydrogenation hydration F2 80 F3 σ F4 F5 F6 1 ! 2 # 3 $ 4 % 05 Q W & Å © 2025 McGraw Hill LLC. All Rights Reserved. F7 F8 ( 6 7 8 9 LU E R T Y U A F9arrow_forwardShow the mechanism steps to obtain the lowerenergy intermediate: *see imagearrow_forwardSoap is made by the previous reaction *see image. The main difference between one soap and another soap isthe length (number of carbons) of the carboxylic acid. However, if a soap irritates your skin, they mostlikely used too much lye.Detergents have the same chemical structure as soaps except for the functional group. Detergentshave sulfate (R-SO4H) and phosphate (R-PO4H2) functional groups. Draw the above carboxylic acidcarbon chain but as the two variants of detergents. *see imagearrow_forward
- Assign all the signals individually (please assign the red, green and blue)arrow_forwardThe two pKa values of oxalic acid are 1.25 and 3.81. Why are they not the same value? Show the protontransfer as part of your explanation. *see imagearrow_forwardасть Identify all the bonds that gauche interact with C-OMe in the most stable conformation of the above compound.arrow_forward
- Predict the reactants used in the formation of the following compounds using Acid-Catalyzed dehydration reactionarrow_forwardCan I please get help with this?arrow_forward.. Give the major organic product(s) for each of the following reactions or sequences of reactions. Show ll relevant stereochemistry [3 ONLY]. A H Br 1. NaCN 2 NaOH, H₂O, heat 3. H3O+ B. CH₂COOH 19000 1. LiAlH4 THF, heat 2 H₂O* C. CH Br 1. NaCN, acetone 2 H3O+, heat D. Br 1. Mg. ether 3. H₂O+ 2 CO₂ E. CN 1. (CH) CHMgBr, ether 2 H₂O+arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY