
Concept explainers
(a)
Interpretation: The reagent for the given conversion is to be interpreted as dilute sulfuric acid or concentrated sulfuric acid.
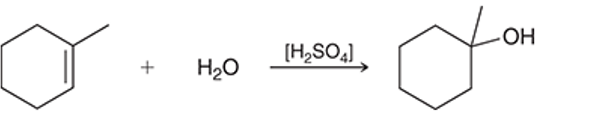
Concept introduction:
(b)
Interpretation: The reagent for the given conversion is to be interpreted as dilute sulfuric acid or concentrated sulfuric acid.
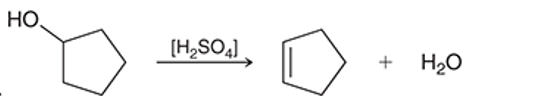
Concept introduction:
Alkenes are unsaturated hydrocarbons with at least one double bond between the carbon atoms. The presence of pi bonds in these molecules makes them more reactive compared to saturated hydrocarbons; alkanes. The stability of alkenes depends on the substituted groups.
Want to see the full answer?
Check out a sample textbook solution
Chapter 8 Solutions
ORGANIC CHEMISTRY-PRINT (LL)-W/WILEY
- Please help me answer a. Please and thank you I advance.arrow_forwardDraw both of the chair flips for both the cis and trans isomers for the following compounds: 1,4-diethylcyclohexane 1-methyl-3-secbutylcyclohexanearrow_forwardPpplllleeeaaasssseeee hellppp wiithhh thisss physical chemistryyyyy I talked like this because AI is very annoyingarrow_forward
- For this question, if the product is racemic, input both enantiomers in the same Marvin editor. A) Input the number that corresponds to the reagent which when added to (E)-but-2-ene will result in a racemic product. Input 1 for Cl, in the cold and dark Input 2 for Oy followed by H₂O, Zn Input 3 for D₂ with metal catalyst Input 4 for H₂ with metal catalyst B) Draw the skeletal structure of the major organic product made from the reagent in part A Marvin JS Help Edit drawing C) Draw the skeletal structure of the major organic product formed when (2)-but-2-ene is treated with peroxyacetic acid. Marvin 35 Helparrow_forwardMichael Reactions 19.52 Draw the products from the following Michael addition reactions. 1. H&C CH (a) i 2. H₂O* (b) OEt (c) EtO H₂NEt (d) ΕΙΟ + 1. NaOEt 2. H₂O' H H 1. NaOEt 2. H₂O*arrow_forwardRank the labeled protons (Ha-Hd) in order of increasing acidity, starting with the least acidic. НОН НЬ OHd Онсarrow_forward
- Can the target compound at right be efficiently synthesized in good yield from the unsubstituted benzene at left? ? starting material target If so, draw a synthesis below. If no synthesis using reagents ALEKS recognizes is possible, check the box under the drawing area. Be sure you follow the standard ALEKS rules for submitting syntheses. + More... Note for advanced students: you may assume that you are using a large excess of benzene as your starting material. C :0 T Add/Remove step Garrow_forwardThe following equations represent the formation of compound MX. What is the AH for the electron affinity of X (g)? X₂ (g) → 2X (g) M (s) → M (g) M (g) M (g) + e- AH = 60 kJ/mol AH = 22 kJ/mol X (g) + e-X (g) M* (g) +X (g) → MX (s) AH = 118 kJ/mol AH = ? AH = -190 kJ/mol AH = -100 kJ/mol a) -80 kJ b) -30 kJ c) -20 kJ d) 20 kJ e) 156 kJarrow_forwardA covalent bond is the result of the a) b) c) d) e) overlap of two half-filled s orbitals overlap of a half-filled s orbital and a half-filled p orbital overlap of two half-filled p orbitals along their axes parallel overlap of two half-filled parallel p orbitals all of the abovearrow_forward
- Can the target compound at right be efficiently synthesized in good yield from the unsubstituted benzene at left? starting material target If so, draw a synthesis below. If no synthesis using reagents ALEKS recognizes is possible, check the box under the drawing area. Be sure you follow the standard ALEKS rules for submitting syntheses. + More... Note for advanced students: you may assume that you are using a large excess of benzene as your starting material. C T Add/Remove step X ноarrow_forwardWhich one of the following atoms should have the largest electron affinity? a) b) c) d) 으으 e) 1s² 2s² 2p6 3s¹ 1s² 2s² 2p5 1s² 2s² 2p 3s² 3p² 1s² 2s 2p 3s² 3p6 4s2 3ds 1s² 2s² 2p6arrow_forwardAll of the following are allowed energy levels except _. a) 3f b) 1s c) 3d d) 5p e) 6sarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning


