
Concept explainers
(a)
Interpretation:
The major product obtained in the given reaction has to be identified.
Concept Introduction:
Markovnikov’s rule: An unsymmetrical

Anti-Markovnikov’s rule: In the presence of per acid an unsymmetrical alkene reacts with hydrogen halide in which halide ions goes to the less substitution position of carbon-carbon double bond which provides alkyl halides.
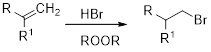
(b)
Interpretation:
The major product obtained in the given reaction has to be identified.
Concept Introduction:
Markovnikov’s rule: An unsymmetrical alkene reacts with hydrogen halide in which halide ions goes to the more substitution position of carbon-carbon double bond which provides alkyl halides.

Anti-Markovnikov’s rule: In the presence of per acid an unsymmetrical alkene reacts with hydrogen halide in which halide ions goes to the less substitution position of carbon-carbon double bond which provides alkyl halides.
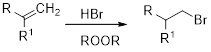
(c)
Interpretation:
The major product obtained in the given reaction has to be identified.
Concept Introduction:
Markovnikov’s rule: An unsymmetrical alkene reacts with hydrogen halide in which halide ions goes to the more substitution position of carbon-carbon double bond which provides alkyl halides.

Anti-Markovnikov’s rule: In the presence of per acid an unsymmetrical alkene reacts with hydrogen halide in which halide ions goes to the less substitution position of carbon-carbon double bond which provides alkyl halides.
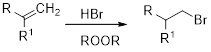
(d)
Interpretation:
The major product obtained in the given reaction has to be identified.
Concept Introduction:
Markovnikov’s rule: An unsymmetrical alkene reacts with hydrogen halide in which halide ions goes to the more substitution position of carbon-carbon double bond which provides alkyl halides.

Anti-Markovnikov’s rule: In the presence of per acid an unsymmetrical alkene reacts with hydrogen halide in which halide ions goes to the less substitution position of carbon-carbon double bond which provides alkyl halides.
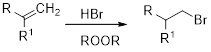
(e)
Interpretation:
The major product obtained in the given reaction has to be identified.
Concept Introduction:
Markovnikov’s rule: An unsymmetrical alkene reacts with hydrogen halide in which halide ions goes to the more substitution position of carbon-carbon double bond which provides alkyl halides.

Anti-Markovnikov’s rule: In the presence of per acid an unsymmetrical alkene reacts with hydrogen halide in which halide ions goes to the less substitution position of carbon-carbon double bond which provides alkyl halides.
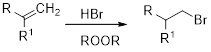
(f)
Interpretation:
The major product obtained in the given reaction has to be identified.
Concept Introduction:
Markovnikov’s rule: An unsymmetrical alkene reacts with hydrogen halide in which halide ions goes to the more substitution position of carbon-carbon double bond which provides alkyl halides.

Anti-Markovnikov’s rule: In the presence of per acid an unsymmetrical alkene reacts with hydrogen halide in which halide ions goes to the less substitution position of carbon-carbon double bond which provides alkyl halides.
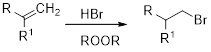
Want to see the full answer?
Check out a sample textbook solution
Chapter 8 Solutions
Organic Chemistry, Third Edition Binder Ready Version
- What alkene or alkyne yields the following products after oxidative cleavage with ozone? Click the "draw structure" button to launch the drawing utility. draw structure ... andarrow_forwardDraw the products of the stronger acid protonating the other reactant. H3C-C=C-4 NH2 KEq CH H3C `CH3 Product acid Product basearrow_forward2. Draw the missing structure(s) in each of the following reactions. The missing structure(s) can be a starting material or the major reaction product(s). C5H10 Br H-Br CH2Cl2 + enant.arrow_forward
- Draw the products of the stronger acid protonating the other reactant. KEq H₂C-O-H H3C OH Product acid Product basearrow_forwardDraw the products of the stronger acid protonating the other reactant. OH KEq CH H3C H3C `CH3 Product acid Product basearrow_forward2. Draw the missing structure(s) in each of the following reactions. The missing structure(s) can be a starting material or the major reaction product(s). Ph H-I CH2Cl2arrow_forward
- 3 attempts left Check my work Draw the products formed in the following oxidative cleavage. [1] 03 [2] H₂O draw structure ... lower mass product draw structure ... higher mass productarrow_forward2. Draw the missing structure(s) in each of the following reactions. The missing structure(s) can be a starting material or the major reaction product(s). H-Br CH2Cl2arrow_forwardWrite the aldol condensation mechanism and product for benzaldehyde + cyclohexanone in a base. Then trans-cinnamaldehyde + acetone in base. Then, trans-cinnamaldehyde + cyclohexanone in a base.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





