
CHEMISTRY:ATOMS FIRST (LL)>CUSTOM PKG.<
2nd Edition
ISBN: 9781259382307
Author: Burdge
Publisher: MCGRAW-HILL HIGHER EDUCATION
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 8.3, Problem 6PPC
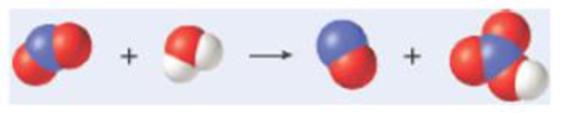
The models here represent the reaction of nitrogen dioxide with water to form nitrogen monoxide and nitric acid. What mass of nitrogen dioxide must react for 100.0 g HNO3 to be produced? (Don’t forget to balance the equation.)
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Using the following two half-reactions, determine the pH range in which $NO_2^-\ (aq)$ cannot be found as the predominant chemical species in water.* $NO_3^-(aq)+10H^+(aq)+8e^-\rightarrow NH_4^+(aq)+3H_2O(l),\ pE^{\circ}=14.88$* $NO_2^-(aq)+8H^+(aq)+6e^-\rightarrow NH_4^+(aq)+2H_2O(l),\ pE^{\circ}=15.08$
Indicate characteristics of oxodec acid.
What is the final product when hexanedioic acid reacts with 1º PCl5 and 2º NH3.
Chapter 8 Solutions
CHEMISTRY:ATOMS FIRST (LL)>CUSTOM PKG.<
Ch. 8.1 - Write and balance the chemical equation for the...Ch. 8.1 - Write and balance the chemical equation that...Ch. 8.1 - Prob. 1PPBCh. 8.1 - Prob. 1PPCCh. 8.1 - Butyric acid (also known as butanoic acid,...Ch. 8.1 - Another compound found in milk fat that appears to...Ch. 8.1 - Prob. 2PPBCh. 8.1 - Prob. 2PPCCh. 8.1 - Prob. 8.3WECh. 8.1 - Prob. 3PPA
Ch. 8.1 - Using the chemical species A2, B, and AB, write a...Ch. 8.1 - Prob. 3PPCCh. 8.1 - Prob. 8.1.1SRCh. 8.1 - Prob. 8.1.2SRCh. 8.1 - Prob. 8.1.3SRCh. 8.1 - Prob. 8.1.4SRCh. 8.1 - Prob. 8.1.5SRCh. 8.2 - Combustion of a 5.50-g sample of benzene produces...Ch. 8.2 - The combustion of a 28.1-g sample of ascorbic acid...Ch. 8.2 - Prob. 4PPBCh. 8.2 - Prob. 4PPCCh. 8.2 - Prob. 8.2.1SRCh. 8.2 - Prob. 8.2.2SRCh. 8.2 - Prob. 8.2.3SRCh. 8.3 - Prob. 8.5WECh. 8.3 - Nitrogen and hydrogen react to form ammonia...Ch. 8.3 - Prob. 5PPBCh. 8.3 - Prob. 5PPCCh. 8.3 - Prob. 8.6WECh. 8.3 - Calculate the mass of water produced by the...Ch. 8.3 - Prob. 6PPBCh. 8.3 - The models here represent the reaction of nitrogen...Ch. 8.3 - Prob. 8.3.1SRCh. 8.3 - Prob. 8.3.2SRCh. 8.3 - Prob. 8.3.3SRCh. 8.3 - Prob. 8.3.4SRCh. 8.4 - Alka-Seltzer tablets contain aspirin, sodium...Ch. 8.4 - Ammonia is produced by the reaction of nitrogen...Ch. 8.4 - Prob. 7PPBCh. 8.4 - The diagrams show a reaction mixture before and...Ch. 8.4 - Aspirin, acetylsalicylic acid (C9H8O4), is the...Ch. 8.4 - Diethyl ether is produced from ethanol according...Ch. 8.4 - What mass of ether will be produced if 207 g of...Ch. 8.4 - The diagrams show a mixture of reactants and the...Ch. 8.4 - Prob. 8.4.1SRCh. 8.4 - Prob. 8.4.2SRCh. 8.4 - Prob. 8.4.3SRCh. 8.4 - Prob. 8.4.4SRCh. 8.4 - Prob. 8.4.5SRCh. 8 - Prob. 8.1QPCh. 8 - Prob. 8.2QPCh. 8 - Why must a chemical equation he balanced? What law...Ch. 8 - Write an unbalanced equation to represent each of...Ch. 8 - Prob. 8.5QPCh. 8 - Prob. 8.6QPCh. 8 - For each of the following unbalanced chemical...Ch. 8 - Prob. 8.8QPCh. 8 - Balance the following equations using the method...Ch. 8 - Which of the following equations best represents...Ch. 8 - Prob. 8.11QPCh. 8 - Determine whether each of the following equations...Ch. 8 - Prob. 8.13QPCh. 8 - Prob. 8.14QPCh. 8 - Prob. 8.15QPCh. 8 - Prob. 8.16QPCh. 8 - Prob. 8.17QPCh. 8 - Prob. 8.18QPCh. 8 - Prob. 8.19QPCh. 8 - Prob. 8.20QPCh. 8 - Prob. 8.21QPCh. 8 - Prob. 8.22QPCh. 8 - Prob. 8.23QPCh. 8 - On what law is stoichiometry based? Why is it...Ch. 8 - Prob. 8.25QPCh. 8 - Prob. 8.26QPCh. 8 - Prob. 8.27QPCh. 8 - Prob. 8.28QPCh. 8 - Prob. 8.29QPCh. 8 - Prob. 8.30QPCh. 8 - Prob. 8.31QPCh. 8 - Prob. 8.32QPCh. 8 - Prob. 8.33QPCh. 8 - When copper(II) sulfate pentahydrate (CuSO4 5H2O)...Ch. 8 - For many years, the extraction of gold from other...Ch. 8 - Prob. 8.36QPCh. 8 - Nitrous oxide (N2O) is also called laughing gas....Ch. 8 - Prob. 8.38QPCh. 8 - Prob. 8.39QPCh. 8 - Prob. 8.1VCCh. 8 - Prob. 8.2VCCh. 8 - Prob. 8.3VCCh. 8 - Prob. 8.4VCCh. 8 - Prob. 8.40QPCh. 8 - Prob. 8.41QPCh. 8 - Why is the theoretical yield of a reaction...Ch. 8 - Why is the actual yield of a reaction almost...Ch. 8 - Prob. 8.44QPCh. 8 - Prob. 8.45QPCh. 8 - Reactants A (red) and B (blue) combine in the...Ch. 8 - Prob. 8.47QPCh. 8 - Prob. 8.48QPCh. 8 - Prob. 8.49QPCh. 8 - Propane (C3H8) is a minor component of natural gas...Ch. 8 - Prob. 8.51QPCh. 8 - Prob. 8.52QPCh. 8 - Prob. 8.53QPCh. 8 - Prob. 8.54QPCh. 8 - Prob. 8.55QPCh. 8 - Prob. 8.56QPCh. 8 - Disulfur dichloride (S2Cl2) is used in the...Ch. 8 - Prob. 8.58QPCh. 8 - Prob. 8.59QPCh. 8 - Prob. 8.60QPCh. 8 - Prob. 8.61QPCh. 8 - Prob. 8.62QPCh. 8 - Prob. 8.63QPCh. 8 - Prob. 8.64QPCh. 8 - Prob. 8.65QPCh. 8 - Industrially, nitric acid is produced by the...Ch. 8 - Prob. 8.67QPCh. 8 - Prob. 8.68QPCh. 8 - Prob. 8.69QPCh. 8 - Prob. 8.70QPCh. 8 - Prob. 8.71QPCh. 8 - Prob. 8.72QPCh. 8 - Prob. 8.73QPCh. 8 - Prob. 8.74QPCh. 8 - Prob. 8.75QPCh. 8 - Prob. 8.76QPCh. 8 - Prob. 8.77QPCh. 8 - Prob. 8.78QPCh. 8 - Prob. 8.79QPCh. 8 - The combustion of a 5.50-g sample of oxalic acid...Ch. 8 - Prob. 8.81QPCh. 8 - Prob. 8.82QPCh. 8 - Prob. 8.83QPCh. 8 - Prob. 8.84QPCh. 8 - Prob. 8.85QPCh. 8 - Prob. 8.86QPCh. 8 - Potash is any potassium mineral that is used for...Ch. 8 - A 21.496-g sample of magnesium is burned in air to...Ch. 8 - Prob. 8.89QPCh. 8 - Prob. 8.90QPCh. 8 - Prob. 8.91QPCh. 8 - Prob. 8.92QPCh. 8 - Prob. 8.93QPCh. 8 - Prob. 8.94QPCh. 8 - Prob. 8.95QPCh. 8 - Prob. 8.96QPCh. 8 - Prob. 8.97QPCh. 8 - Prob. 8.98QPCh. 8 - A compound X contains 63.3 percent manganese (Mn)...Ch. 8 - Calculate the mass of water produced in the...Ch. 8 - Calcium phosphide (Ca3P2) and water react to form...Ch. 8 - Prob. 8.3KSPCh. 8 - Prob. 8.4KSP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the final product when D-galactose reacts with hydroxylamine?arrow_forwardIndicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.arrow_forwardIn the two chair conformations of glucose, the most stable is the one with all the OH groups in the equatorial position. Is this correct?arrow_forward
- please help me with my homeworkarrow_forwardhelparrow_forwardThe temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forward
- QUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forwardpressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forwarder your payment details | bar xb Home | bartleby x + aleksogi/x/isl.exe/1o u-lgNskr7j8P3jH-1Qs_pBanHhviTCeeBZbufuBYT0Hz7m7D3ZcW81NC1d8Kzb4srFik1OUFhKMUXzhGpw7k1 O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 1 3- 0- 0 200 Explanation Check temperature (K) 400 X Q Search L G 2025 McGraw Hill LLC. All Rights Reserved Terms of Use Privacy Cearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY