
Chemistry - Modified MasteringChemistry
7th Edition
ISBN: 9780133892321
Author: McMurry
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 8, Problem 8.9P
PRACTICE 8.9 Describe the hybridization of the carbon atom in carbon dioxide, and make a rough sketch of the molecule showing its hybrid orbitals and 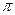 bonds.
bonds.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Some of the theories used to describe interface structure can be distinguished by:1. the measured potential difference.2. the distribution of ions in solution.3. the calculation of charge density.4. the external Helmoltz plane.
When talking about the acidity of carboxylic acids, is it the same thing to say higher or stronger acidity?
Using the following two half-reactions, determine the pH range in which $NO_2^-\ (aq)$ cannot be found as the predominant chemical species in water.* $NO_3^-(aq)+10H^+(aq)+8e^-\rightarrow NH_4^+(aq)+3H_2O(l),\ pE^{\circ}=14.88$* $NO_2^-(aq)+8H^+(aq)+6e^-\rightarrow NH_4^+(aq)+2H_2O(l),\ pE^{\circ}=15.08$
Chapter 8 Solutions
Chemistry - Modified MasteringChemistry
Ch. 8 - Prob. 8.1PCh. 8 - Prob. 8.2ACh. 8 - PRACTICE 8.3 Acetic acid, CH3CO2H , is the main...Ch. 8 - APPLY 8.4 Benzene, C6H6 , is a cyclic molecule in...Ch. 8 - PRACTICE 8.5 Identify the orbitals that overlap to...Ch. 8 - APPLY 8.6 Describe the bonding in propane, C3H8 ,...Ch. 8 - PRACTICE 8.7 Describe the hybridization of the...Ch. 8 - APPLY 8.8 Describe the hybridization of each...Ch. 8 - PRACTICE 8.9 Describe the hybridization of the...Ch. 8 - APPLY 8.10 Describe the hybridization of the...
Ch. 8 - Prob. 8.11PCh. 8 - Conceptual APPLY 8.12 Match the following...Ch. 8 - Prob. 8.13PCh. 8 - Prob. 8.14ACh. 8 - Prob. 8.15PCh. 8 - Prob. 8.16ACh. 8 - Prob. 8.17PCh. 8 - Prob. 8.18ACh. 8 - Prob. 8.19PCh. 8 - Prob. 8.20ACh. 8 - Prob. 8.21PCh. 8 - Prob. 8.22ACh. 8 - PRACTICE 8.23 Draw two resonance structures for...Ch. 8 - APPLY 8.24 Draw two resonance structures for the...Ch. 8 - Prob. 8.25PCh. 8 - Prob. 8.26PCh. 8 - PROBLEM 8.27 Identify which of the following...Ch. 8 - Prob. 8.28PCh. 8 - Prob. 8.29PCh. 8 - Prob. 8.30PCh. 8 - Prob. 8.31CPCh. 8 - Prob. 8.32CPCh. 8 - Prob. 8.33CPCh. 8 - Prob. 8.34CPCh. 8 - Prob. 8.35CPCh. 8 - Prob. 8.36CPCh. 8 - Prob. 8.37CPCh. 8 - Prob. 8.38CPCh. 8 - Prob. 8.39CPCh. 8 - Prob. 8.40CPCh. 8 - Two dichioroethylene molecules with the same...Ch. 8 - Prob. 8.42SPCh. 8 - Prob. 8.43SPCh. 8 - Prob. 8.44SPCh. 8 - How many charge clouds are there around the...Ch. 8 - Prob. 8.46SPCh. 8 - Prob. 8.47SPCh. 8 - What shape do you expect for each of the following...Ch. 8 - Prob. 8.49SPCh. 8 - Prob. 8.50SPCh. 8 - Prob. 8.51SPCh. 8 - Prob. 8.52SPCh. 8 - Prob. 8.53SPCh. 8 - Acrylonitrile is used as the starting material for...Ch. 8 - Predict values for all bond angles in dimethyl...Ch. 8 - Oceanographers study the mixing of water masses by...Ch. 8 - Prob. 8.57SPCh. 8 - Prob. 8.58SPCh. 8 - Prob. 8.59SPCh. 8 - Prob. 8.60SPCh. 8 - Prob. 8.61SPCh. 8 - Prob. 8.62SPCh. 8 - Prob. 8.63SPCh. 8 - Prob. 8.64SPCh. 8 - Prob. 8.65SPCh. 8 - Oxaloacetic acid is an intermediate involved in...Ch. 8 - The atoms in the amino acid glycine are connected...Ch. 8 - Prob. 8.68SPCh. 8 - Prob. 8.69SPCh. 8 - Prob. 8.70SPCh. 8 - 8.71 What is the difference between London...Ch. 8 - 8.72 What are the most important kinds of...Ch. 8 - Of the substances Xe,CH3Cl,HF, which has: (a) The...Ch. 8 - 8.74 Methanol boils nearlyhigher than methane, but...Ch. 8 - Prob. 8.75SPCh. 8 - Prob. 8.76SPCh. 8 - Prob. 8.77SPCh. 8 - Prob. 8.78SPCh. 8 - Prob. 8.79SPCh. 8 - Prob. 8.80SPCh. 8 - 8.81 Draw three-dimensional structures of PCl3 and...Ch. 8 - Prob. 8.82SPCh. 8 - Prob. 8.83SPCh. 8 - Prob. 8.84SPCh. 8 - Prob. 8.85SPCh. 8 - 8.86 A liquid sample contains methylamine (CH3NH2)...Ch. 8 - Prob. 8.87SPCh. 8 - Prob. 8.88SPCh. 8 - Prob. 8.89SPCh. 8 - Prob. 8.90SPCh. 8 - Prob. 8.91SPCh. 8 - Prob. 8.92SPCh. 8 - Prob. 8.93SPCh. 8 - Prob. 8.94SPCh. 8 - Prob. 8.95SPCh. 8 - Prob. 8.96SPCh. 8 - Prob. 8.98CPCh. 8 - Prob. 8.99CPCh. 8 - Prob. 8.100CPCh. 8 - Prob. 8.101CPCh. 8 - Prob. 8.102CPCh. 8 - Prob. 8.103CPCh. 8 - Prob. 8.104CPCh. 8 - Prob. 8.105CPCh. 8 - Prob. 8.106CPCh. 8 - Prob. 8.107CPCh. 8 - Prob. 8.108CPCh. 8 - Prob. 8.109CPCh. 8 - The odor of cinnamon oil is due to cinnamaldehyde,...Ch. 8 - Prob. 8.111CPCh. 8 - Prob. 8.112CPCh. 8 - Prob. 8.113CPCh. 8 - Prob. 8.114CPCh. 8 - Prob. 8.115CPCh. 8 - Prob. 8.116CPCh. 8 - Prob. 8.117CPCh. 8 - Prob. 8.118CPCh. 8 - Prob. 8.119MPCh. 8 - Prob. 8.120MPCh. 8 - Prob. 8.121MP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Indicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.arrow_forwardIn the two chair conformations of glucose, the most stable is the one with all the OH groups in the equatorial position. Is this correct?arrow_forwardIndicate the formula of the product obtained by reacting D-Galactose with hydroxylamine.arrow_forward
- helparrow_forwardThe temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forwardQUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781285199023
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning


Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning
Linear Combination of Atomic Orbitals LCAO; Author: Edmerls;https://www.youtube.com/watch?v=nq1zwrAIr4c;License: Standard YouTube License, CC-BY
Quantum Molecular Orbital Theory (PChem Lecture: LCAO and gerade ungerade orbitals); Author: Prof Melko;https://www.youtube.com/watch?v=l59CGEstSGU;License: Standard YouTube License, CC-BY