
Interpretation:
The ions from
Concept introduction:
Paramagnetism is a form of magnetism where the materials are weakly attracted by an externally applied magnetic field. It is due to the presence of unpaired electrons in the materials so all the atoms with incompletely filled atomic orbital are paramagnetic.
The intensity of paramagnetism increases with the increase in the number of unpaired electrons. Due to their spin, the unpaired electrons have a magnetic dipole moment and act like tiny magnets.
Answer to Problem 8.97P
Explanation of Solution
The paramagnetic nature of the ions of iron can be determined by the presence of unpaired electrons. The
The condensed electronic configuration of
Its partial orbital diagram is as follows:
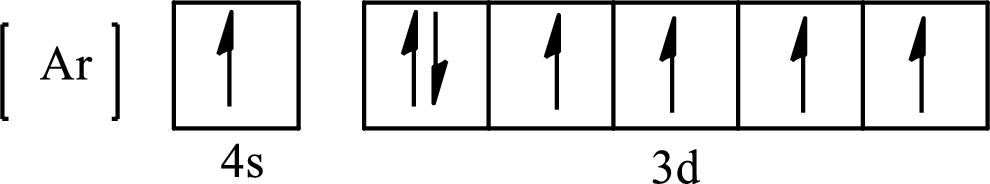
The condensed electronic configuration of
Its partial orbital diagram is as follows:
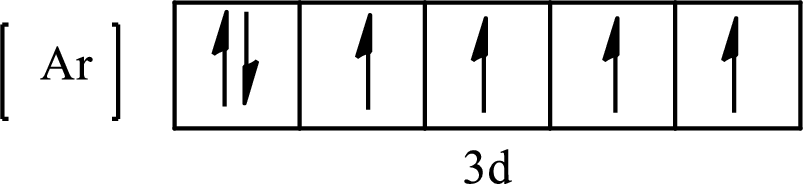
The condensed electronic configuration of
Its partial orbital diagram is as follows:
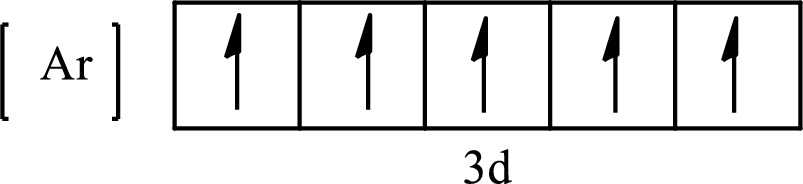
The condensed electronic configuration of
Its partial orbital diagram is as follows:
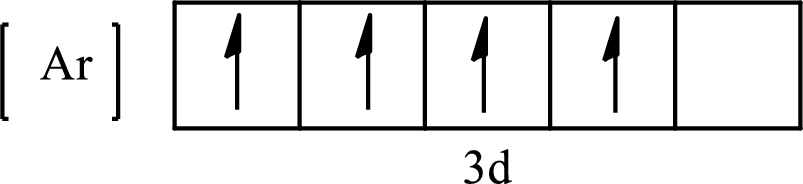
The condensed electronic configuration of
Its partial orbital diagram is as follows:
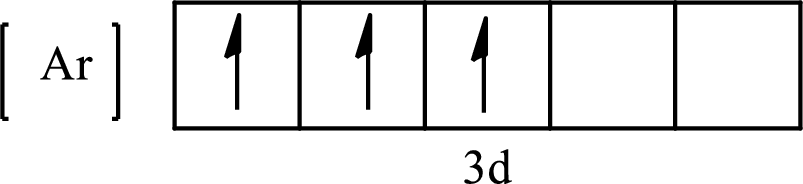
The condensed electronic configuration of
Its partial orbital diagram is as follows:
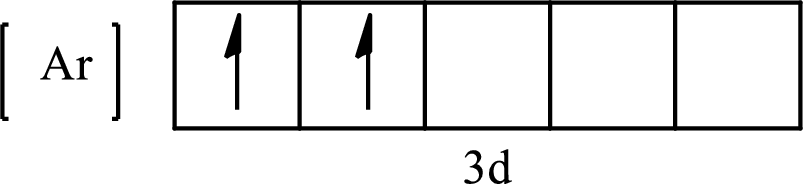
The condensed electronic configuration of
Its partial orbital diagram is as follows:
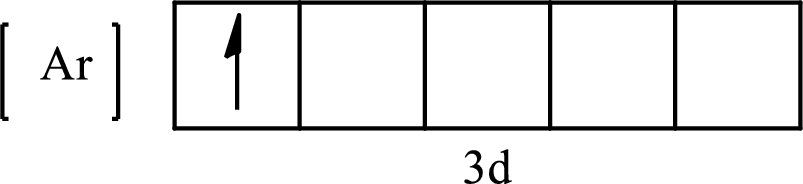
The condensed electronic configuration of
Its partial orbital diagram is as follows:
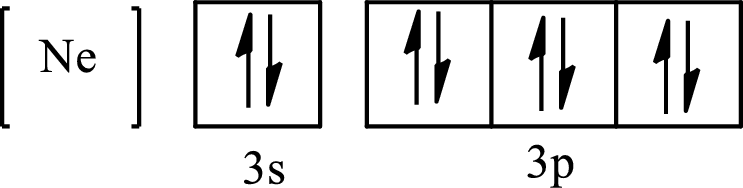
The condensed electronic configuration of
Its partial orbital diagram is as follows:
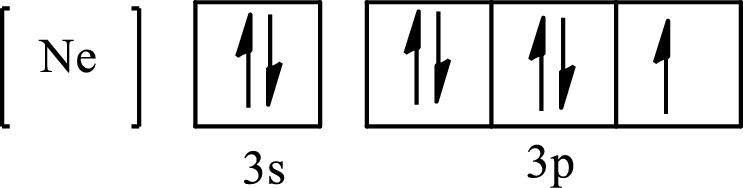
The condensed electronic configuration of
Its partial orbital diagram is as follows:
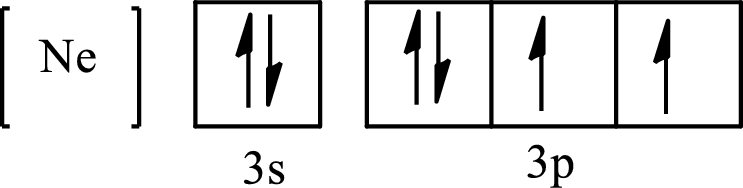
The condensed electronic configuration of
Its partial orbital diagram is as follows:
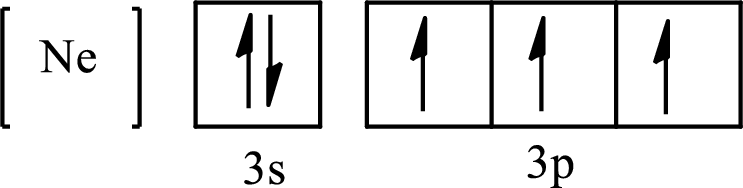
The condensed electronic configuration of
Its partial orbital diagram is as follows:
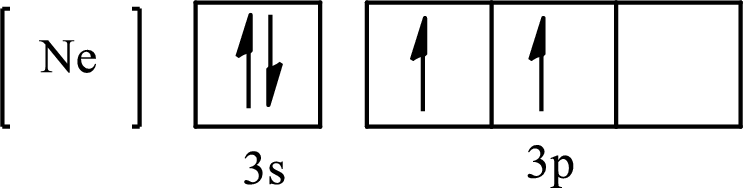
The condensed electronic configuration of
Its partial orbital diagram is as follows:
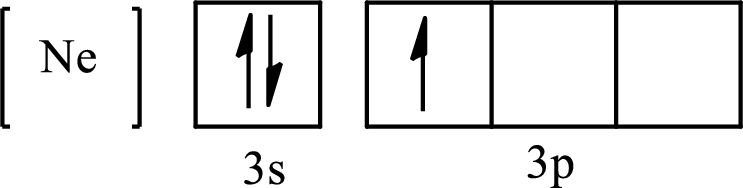
The condensed electronic configuration of
Its partial orbital diagram is as follows:
Want to see more full solutions like this?
Chapter 8 Solutions
CHEMISTRY >CUSTOM<
- When anisole is treated with excess bromine, the reaction gives a product which shows two singlets in 1H NMR. Draw the product.arrow_forward(ii) Draw a reasonable mechanism for the following reaction: CI NaOH heat OH (hint: SNAr Reaction) :arrow_forwardDraw the major product in each of the following reaction:arrow_forward
- Draw the mechanism for the following Friedel-Craft reaction. AlBr3 Brarrow_forward(a) Draw the structures of A and B in the following reaction. (i) NaNH2, NH3(1) A + B (ii) H3O+arrow_forwardFor the reaction 2 N2O5(g) → 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 →> NO₂+ NO3_(K1) NO2 + NO3 → N2O5 (k-1) NO2 + NO3 → → NO2 + O2 + NO (K2) NO + N2O5- NO2 + NO2 + NO2 (K3) d[N₂O5] __2k‚k₂[N2O5] Indicate whether the following rate expression is acceptable: dt k₁₁+ k₂arrow_forward
- Consider the following decomposition reaction of N2O5(g): For the reaction 2 N2O5(g) → 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 → NO2 + NO3 (K1) NO2 + NO3 → N2O5 (k-1) NO2 + NO3 → NO2 + O2 + NO (K2) NO + N2O5 → NO2 + NO2 + NO2 (K3) Indicate whether the following rate expression is acceptable: d[N2O5] = -k₁[N₂O₂] + K¸₁[NO₂][NO3] - K¸[NO₂]³ dtarrow_forwardIn a reaction of A + B to give C, another compound other than A, B or C may appear in the kinetic equation.arrow_forwardFor the reaction 2 N2O5(g) → 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 →> NO₂+ NO3_(K1) NO2 + NO3 → N2O5 (k-1) NO2 + NO3 → → NO2 + O2 + NO (K2) NO + N2O5- NO2 + NO2 + NO2 (K3) d[N₂O5] __2k‚k₂[N2O5] Indicate whether the following rate expression is acceptable: dt k₁₁+ k₂arrow_forward
- Given the reaction R + Q → P, indicate the rate law with respect to R, with respect to P and with respect to P.arrow_forwardSteps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forwardk₁ Given the reaction A B, indicate k-1 d[A] (A). the rate law with respect to A: (B). the rate law with respect to B: d[B] dt dtarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





