
(a)
Interpretation:
The mechanism for the given elimination reaction including carbocation rearrangement is to be drawn.
Concept introduction:
The
Answer to Problem 8.65P
The
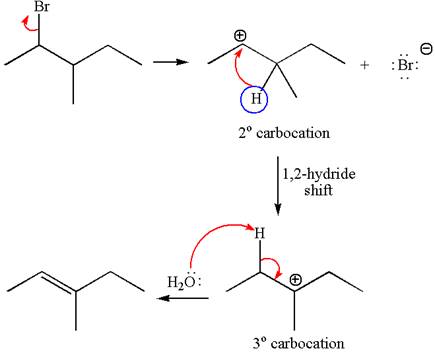
Explanation of Solution
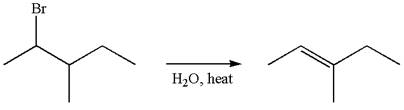
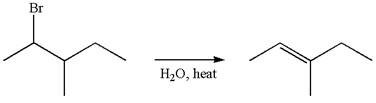
The given reaction equation is
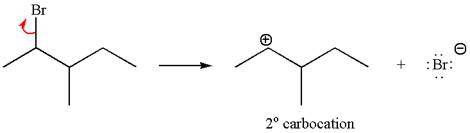
In first step, the leaving group
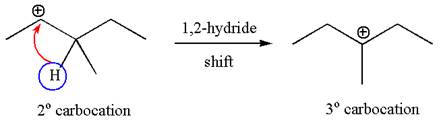
The carbocation formed is rearranged by
The water molecule acts as a base and abstracts a proton from the carbon adjacent to the carbocation, forming
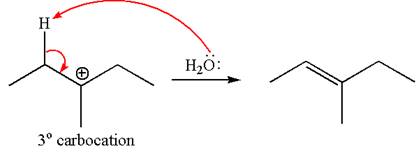
The mechanism for the given elimination reaction is drawn to show the carbocation rearrangement by
(b)
Interpretation:
The mechanism for the given elimination reaction without carbocation rearrangement is to be drawn.
Concept introduction:
The
Answer to Problem 8.65P
The
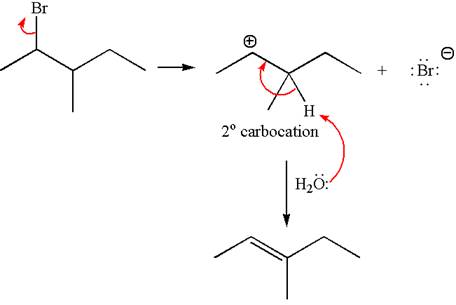
Explanation of Solution
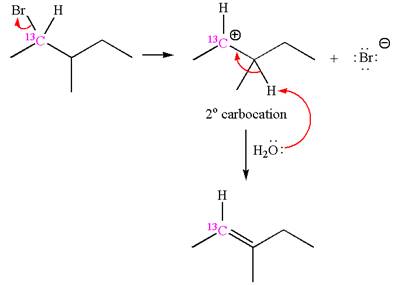
The given reaction equation is
In the first step, the leaving group
In the second step, without rearrangement, the proton is eliminated by the base
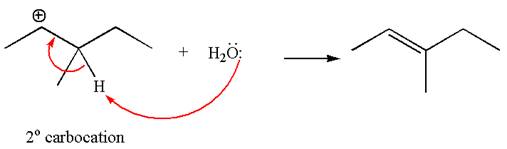
The mechanism for the given elimination reaction is drawn to without rearrangement step, indicating that the same product is formed with or without rearrangement.
(c)
Interpretation:
It is to be explained how the
Concept introduction:
The
Answer to Problem 8.65P
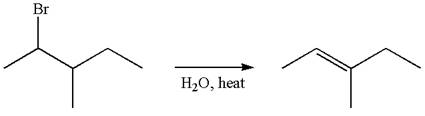
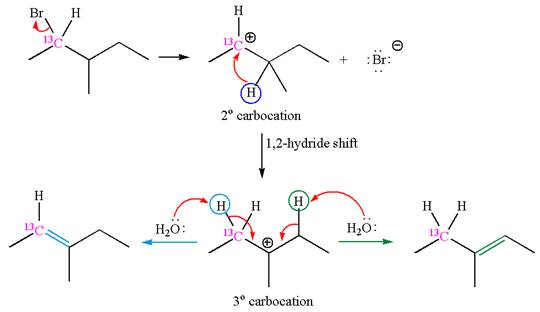
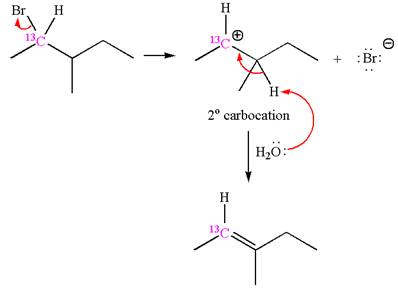
The reaction with carbocation rearrangement gave two products, and the reaction without carbocation rearrangement gave only one product, as shown below, indicating that the
Reaction with rearrangement:
Reaction without rearrangement:
Explanation of Solution
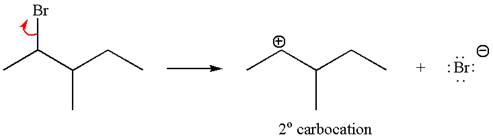
The given reaction equation is
If the carbon bonded to the leaving group in the given substrate is labelled as
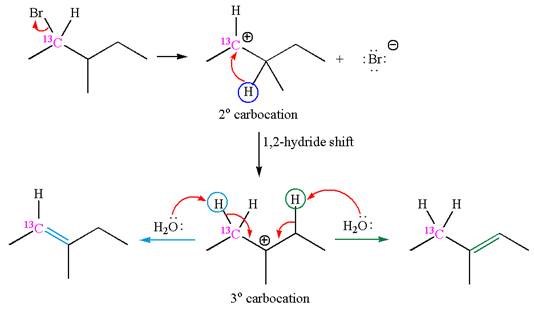
In one product, one of the double bonded carbon is
If the reaction proceeds without rearrangement, then only one product is formed where one of the double bonded carbon is
As the reaction with rearrangement of carbocation formed two products, and reaction without rearrangement formed only one product, it indicates that the E1 products depend on whether the rearrangement occurred.
It is explained that the E1 products depend on whether the reaction includes carbocation rearrangement occurring with
(d)
Interpretation:
How the deuterium isotope labeling is useful to determine whether the rearrangement is occurred in given
Concept introduction:
The
Answer to Problem 8.65P
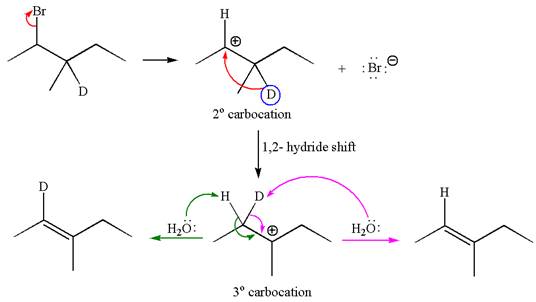
The reaction with carbocation rearrangement by migration of deuterium gave two products, and the reaction without carbocation rearrangement gave only one product as shown below, indicating that the deuterium isotope labeling is useful to determine whether the rearrangement occurred in the given
Reaction with rearrangement:
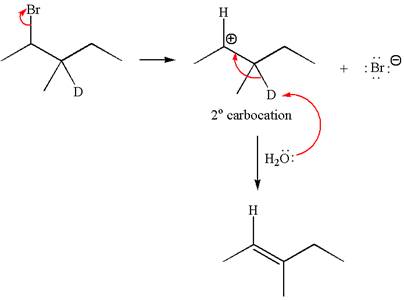
Reaction without rearrangement:
Explanation of Solution
The given reaction equation is
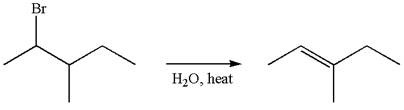
If the migrating hydrogen in the given substrate is replaced with deuterium, then two products are formed when the reaction occurred through carbocation rearrangement. The detailed mechanism is as follows:
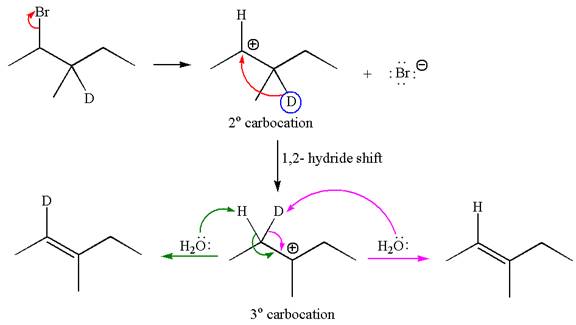
One product is formed by elimination of hydrogen atom and another by elimination of deuterium atom.
If the reaction proceeds without rearrangement, only one product is formed by elimination of deuterium atom. The detailed mechanism is as follows:
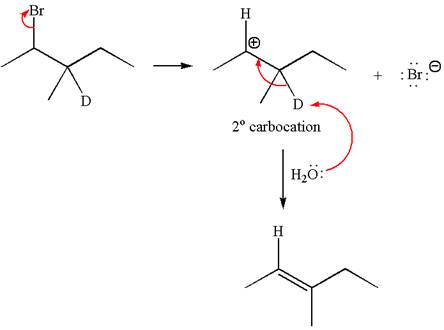
As the reaction with rearrangement of carbocation formed two products and reaction without rearrangement formed only one product, it indicates the E1 products depend on whether the rearrangement occurs.
It is explained on the basis of formation of different products that deuterium isotope labeling is useful to determine whether the rearrangement occurred in the given
Want to see more full solutions like this?
Chapter 8 Solutions
Organic Chemistry: Principles And Mechanisms
- Complete the following esterification reaction by drawing the structural formula of the product formed. HOH HO i catalyst catalyst OH HO (product has rum flavor) (product has orange flavor)arrow_forwardThe statements in the tables below are about two different chemical equilibria. The symbols have their usual meaning, for example AG stands for the standard Gibbs free energy of reaction and K stands for the equilibrium constant. In each table, there may be one statement that is faise because it contradicts the other three statements. If you find a false statement, check the box next to t Otherwise, check the "no false statements" box under the table. statement false? AG"1 no false statements: statement false? AG-0 0 InK-0 0 K-1 0 AH-TAS no false statements 2arrow_forwardComplete the following esterification reactions by drawing the line formulas of the carboxylic acid and alcohol required to form the ester shown. catalyst catalyst catalyst apricot fragrancearrow_forward
- Show the saponification products of the following ester: You don't need to draw in the Na+ cation. catalyst, A catalyst, A catalyst, Aarrow_forwardWhat would happen if the carboxylic acid and alcohol groups were on the same molecule? In essence, the molecule reacts with itself. Draw the structure of the products formed in this manner using the reactants below. If two functional groups interact with one another on the same molecule, this is called an “intramolecular" (within one) rather than "intermolecular" (between two or more) attack. OH OH catalyst OH HO catalyst catalyst HO OHarrow_forwardQ3: Write in the starting alkyl bromide used to form the following products. Include any reactants, reagents, and solvents over the reaction arrow. If more than one step is required, denote separate steps by using 1), 2), 3), etc. H OH racemic OH OH 5 racemicarrow_forward
- Draw the Lewis structure of the SO3-O(CH3)2 complex shown in the bottom right of slide 2in lecture 3-3 (“Me” means a CH3 group) – include all valence electron pairs and formal charges.From this structure, should the complex be a stable molecule? Explain.arrow_forwardPredict all organic product(s), including stereoisomers when applicable.arrow_forwardQ5: Propose a reasonable synthesis for the following decalin derivative. using only decalin and alkanes of 3 or fewer carbons. Decalin H3C HO க CH3arrow_forward
- 2Helparrow_forwardplease add appropriate arrows, and tell me clearly where to add arrows, or draw itarrow_forwardWhat I Have Learned Directions: Given the following reaction and the stress applied in each reaction, answer the question below. A. H2(g) + Cl2(g) 2 HCl(g) Stress applied: Decreasing the pressure 1. What is the Keq expression? 2. What will be the effect in the number of moles of HCl(g)? 3. What will be the Equilibrium Shift or the reaction? B. Fe3O4(s) + 4 H2(g) + heat 53 Fe(s) + 4 H₂O(g) Stress applied: Increasing the temperature 1. What is the Keq expression?. 2. What will be the effect in the volume of water vapor collected? 3. What will be the Equilibrium Shift or the reaction? C. 4 NH3(g) + 5 O2(g) 4 NO(g) + 6 H2O(g) + heat Stress applied: Increasing the volume of the container 1. What is the Keq expression?. 2. What will be the effect in the amount of H₂O? 3. What will be the Equilibrium Shift or the reaction?arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
