
EBK CHEMISTRY
12th Edition
ISBN: 9780133911312
Author: Timberlake
Publisher: YUZU
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 8, Problem 8.56UTC
Indicate which diagram (1, 2, or 3) represents the volume of the gas sample in a flexible container when each of the following changes (a to d) takes place: (8.2, 8.3)
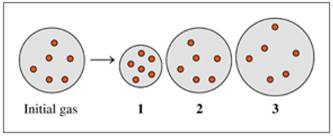
- Temperature increases if pressure does not change.
- Temperature decreases if pressure does not change.
- Atmospheric pressure decreases if temperature does not change.
- Doubling the atmospheric pressure and doubling the Kelvin temperature.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Complete the following synthesis.
(d). H+
ง
с
Can the target compound be efficiently synthesized in good yield from the substituted benzene of the starting material?
If yes, draw the synthesis. Include all steps and all reactants.
This is a synthesis question. Why is this method wrong or worse than the "correct" method? You could do it thiss way, couldn't you?
Chapter 8 Solutions
EBK CHEMISTRY
Ch. 8.1 - Prob. 8.1QAPCh. 8.1 - Use the kinetic molecular theory of gases to...Ch. 8.1 - Identify the property of a gas that is measured in...Ch. 8.1 - Identify the property of a gas that is measured in...Ch. 8.1 - Prob. 8.5QAPCh. 8.1 - Prob. 8.6QAPCh. 8.1 - Prob. 8.7QAPCh. 8.1 - Prob. 8.8QAPCh. 8.2 - Why do scuba divers need to exhale air when they...Ch. 8.2 - Why does a sealed bag of chips expand when you...
Ch. 8.2 - Prob. 8.11QAPCh. 8.2 - A balloon is filled with helium gas. When each of...Ch. 8.2 - Prob. 8.13QAPCh. 8.2 - Prob. 8.14QAPCh. 8.2 - A 10.0-L balloon contains helium gas at a pressure...Ch. 8.2 - Prob. 8.16QAPCh. 8.2 - A sample of nitrogen N2 has a volume of 50.0 L at...Ch. 8.2 - A sample of methane CH4 has a volume of 25 mL at a...Ch. 8.2 - Prob. 8.19QAPCh. 8.2 - Prob. 8.20QAPCh. 8.2 - Prob. 8.21QAPCh. 8.2 - Prob. 8.22QAPCh. 8.3 - Select the diagram that shows the final volume of...Ch. 8.3 - Indicate whether the final volume of gas in each...Ch. 8.3 - A sample of neon initially has a volume of 2.50 L...Ch. 8.3 - Prob. 8.26QAPCh. 8.3 - A balloon contains 2500 ml- of helium gas at 75C ....Ch. 8.3 - Prob. 8.28QAPCh. 8.4 - Prob. 8.29QAPCh. 8.4 - Prob. 8.30QAPCh. 8.4 - Calculate the final temperature, in degrees...Ch. 8.4 - Calculate the final temperature, in degrees...Ch. 8.5 - Prob. 8.33QAPCh. 8.5 - A sample of argon gas has a volume of 735 mL at a...Ch. 8.5 - A 124-mL bubble of hot gas initially at 212 °C and...Ch. 8.5 - A scuba diver 60 ft below the ocean surface...Ch. 8.6 - What happens to the volume of a bicycle tire or a...Ch. 8.6 - Prob. 8.38QAPCh. 8.6 - Prob. 8.39QAPCh. 8.6 - Prob. 8.40QAPCh. 8.6 - Prob. 8.41QAPCh. 8.6 - Prob. 8.42QAPCh. 8.6 - Prob. 8.43QAPCh. 8.6 - Prob. 8.44QAPCh. 8.7 - A typical air sample in the lungs contains oxygen...Ch. 8.7 - Prob. 8.46QAPCh. 8.7 - Prob. 8.47QAPCh. 8.7 - In a gas mixture, the partial pressures are argon...Ch. 8.7 - A gas mixture containing oxygen, nitrogen, and...Ch. 8.7 - A gas mixture containing oxygen, nitrogen, and...Ch. 8.7 - Prob. 8.51QAPCh. 8.7 - Prob. 8.52QAPCh. 8 - Two flasks of equal volume and at the same...Ch. 8 - Prob. 8.54UTCCh. 8 - At 100 °C, which of the following diagrams (1, 2,...Ch. 8 - Indicate which diagram (1, 2, or 3) represents the...Ch. 8 - A balloon is filled with helium gas with a partial...Ch. 8 - Prob. 8.58UTCCh. 8 - Prob. 8.59UTCCh. 8 - Prob. 8.60UTCCh. 8 - Prob. 8.61AQAPCh. 8 - Prob. 8.62AQAPCh. 8 - Prob. 8.63AQAPCh. 8 - Prob. 8.64AQAPCh. 8 - A weather balloon has a volume of 750 L when...Ch. 8 - Prob. 8.66AQAPCh. 8 - A weather balloon is partially filled with Helium...Ch. 8 - Prob. 8.68AQAPCh. 8 - A gas mixture contains Oxygen and Argon at partial...Ch. 8 - Prob. 8.70AQAPCh. 8 - Prob. 8.71CQCh. 8 - You are doing research on planet X. The...Ch. 8 - Prob. 8.73CQCh. 8 - Prob. 8.74CQCh. 8 - Prob. 8.75CQCh. 8 - Prob. 8.76CQ
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Try: Draw the best Lewis structure showing all non-bonding electrons and all formal charges if any: (CH3)3CCNO NCO- HN3 [CH3OH2]*arrow_forwardWhat are the major products of the following reaction? Draw all the major products. If there are no major products, then there is no reaction that will take place. Use wedge and dash bonds when necessary.arrow_forwardZeolites. State their composition and structure. Give an example.arrow_forward
- Don't used hand raiting and show all reactionsarrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forwardIX) By writing the appropriate electron configurations and orbital box diagrams briefly EXPLAIN in your own words each one of the following questions: a) The bond length of the Br2 molecule is 2.28 Å, while the bond length of the compound KBr is 3.34 Å. The radius of K✶ is 1.52 Å. Determine the atomic radius in Å of the bromine atom and of the bromide ion. Br = Br b) Explain why there is a large difference in the atomic sizes or radius of the two (Br and Br). Tarrow_forward
- When 15.00 mL of 3.00 M NaOH was mixed in a calorimeter with 12.80 mL of 3.00 M HCl, both initially at room temperature (22.00 C), the temperature increased to 29.30 C. The resultant salt solution had a mass of 27.80 g and a specific heat capacity of 3.74 J/Kg. What is heat capacity of the calorimeter (in J/C)? Note: The molar enthalpy of neutralization per mole of HCl is -55.84 kJ/mol.arrow_forwardWhen 15.00 mL of 3.00 M NaOH was mixed in a calorimeter with 12.80 mL of 3.00 M HCl, both initially at room temperature (22.00 C), the temperature increased to 29.30 C. The resultant salt solution had a mass of 27.80 g and a specific heat capacity of 3.74 J/Kg. What is heat capacity of the calorimeter (in J/C)? Note: The molar enthalpy of neutralization per mole of HCl is -55.84 kJ/mol. Which experimental number must be initialled by the Lab TA for the first run of Part 1 of the experiment? a) the heat capacity of the calorimeter b) Mass of sample c) Ti d) The molarity of the HCl e) Tfarrow_forwardPredict products for the Following organic rxn/s by writing the structurels of the correct products. Write above the line provided" your answer D2 ①CH3(CH2) 5 CH3 + D₂ (adequate)" + 2 mited) 19 Spark Spark por every item. 4 CH 3 11 3 CH 3 (CH2) 4 C-H + CH3OH CH2 CH3 + CH3 CH2OH 0 CH3 fou + KMnDy→ C43 + 2 KMn Dy→→ C-OH ") 0 C-OH 1110 (4.) 9+3 =C CH3 + HNO 3 0 + Heat> + CH3 C-OH + Heat CH2CH3 - 3 2 + D Heat H 3 CH 3 CH₂ CH₂ C = CH + 2 H₂ → 2 2arrow_forward
- When 15.00 mL of 3.00 M NaOH was mixed in a calorimeter with 12.80 mL of 3.00 M HCl, both initially at room temperature (22.00 C), the temperature increased to 29.30 C. The resultant salt solution had a mass of 27.80 g and a specific heat capacity of 3.74 J/Kg. What is heat capacity of the calorimeter (in J/C)? Note: The molar enthalpy of neutralization per mole of HCl is -55.84 kJ/mol.arrow_forwardQ6: Using acetic acid as the acid, write the balanced chemical equation for the protonation of the two bases shown (on the -NH2). Include curved arrows to show the mechanism. O₂N- O₂N. -NH2 -NH2 a) Which of the two Bronsted bases above is the stronger base? Why? b) Identify the conjugate acids and conjugate bases for the reactants. c) Identify the Lewis acids and bases in the reactions.arrow_forwardQ5: For the two reactions below: a) Use curved electron-pushing arrows to show the mechanism for the reaction in the forward direction. Redraw the compounds to explicitly illustrate all bonds that are broken and all bonds that are formed. b) Label Bronsted acids and bases in the left side of the reactions. c) For reaction A, which anionic species is the weakest base? Which neutral compound is the stronger acid? Is the forward or reverse reaction favored? d) Label Lewis acids and bases, nucleophiles and electrophiles in the left side of the reactions. A. 용 CH3OH я хон CH3O OH B. HBr CH3ONa NaBr CH3OHarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning
Calorimetry Concept, Examples and Thermochemistry | How to Pass Chemistry; Author: Melissa Maribel;https://www.youtube.com/watch?v=nSh29lUGj00;License: Standard YouTube License, CC-BY