
Concept explainers
(a)
Interpretation:
Whether a carbocation rearrangement will take place in an
Concept introduction:
The first step in an
A carbocation may undergo rearrangement through a
Stability of carbocations increases as
Answer to Problem 8.52P
A carbocation rearrangement will take place for this substrate.
The rearrangement can be drawn using curved arrow notation as
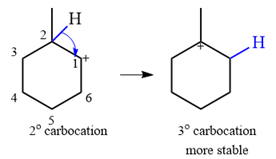
Explanation of Solution
The given substrate initially forms the carbocation shown below in an
It is a relatively stable secondary carbocation. It will undergo a rearrangement only if it leads to the formation of a more stable tertiary carbocation. Two
Therefore, a carbocation rearrangement is possible in this case.
The rearrangement can be drawn using the curved arrow notation as
Formation of a more stable tertiary carbocation results in the rearrangement for this substrate.
(b)
Interpretation:
Whether a carbocation rearrangement will take place in an
Concept introduction:
The first step in an
A carbocation may undergo rearrangement through a
Stability of carbocations increases as
Answer to Problem 8.52P
There will be no carbocation rearrangement in the case of this substrate.
Explanation of Solution
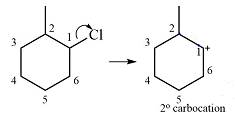
The substrate and the carbocation that it will be formed initially in an
The carbocation initially formed is a relatively stable secondary carbocation. There are two hydrogen atoms on adjacent carbons (C2 and C6) that can undergo a
Therefore, a carbocation rearrangement will not take place in this case.
There is no carbocation rearrangement for this case as there is no gain in stability.
(c)
Interpretation:
Whether a carbocation rearrangement will take place in an
Concept introduction:
The first step in an
A carbocation may undergo rearrangement through a
Stability of carbocations increases as
Answer to Problem 8.52P
A carbocation rearrangement will not take place in this case.
Explanation of Solution
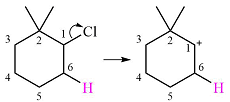
The given substrate initially forms the carbocation shown below in an
The carbocation formed is a tertiary carbocation, the most stable one. Therefore, a rearrangement will occur only if it leads to another tertiary carbocation that is further stabilized by resonance. No resonance stabilization is possible here as there are no double bonds in the carbocation.
Therefore, a carbocation rearrangement will not take place in this case.
Carbocation rearrangement is not possible because the one initially formed is a tertiary carbocation.
(d)
Interpretation:
Whether a carbocation rearrangement will take place in an
Concept introduction:
The first step in an
A carbocation may undergo rearrangement through a
Stability of carbocations increases as
Answer to Problem 8.52P
A carbocation rearrangement will take place in this case.
The curved arrow notation for the rearrangement can be drawn as
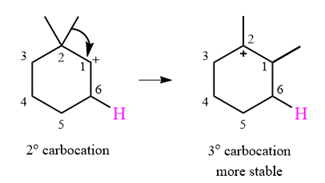
Explanation of Solution
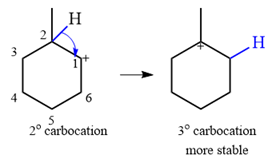
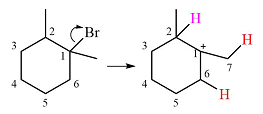
The substrate shown and the carbocation that it will initially form in this case is
The carbocation initially formed is a relatively stable secondary carbocation. It will undergo a rearrangement only if it leads to the formation of a resonance stabilized secondary carbocation or a tertiary carbocation. Only one hydride shift is possible, but it will not occur as the carbocation formed will be a similar secondary carbocation.
A methyl shift, from C2 to C1 will, however, lead to the formation of a tertiary carbocation.
Therefore, a carbocation rearrangement will take place in this case.
The curved arrow notation for this rearrangement can be drawn as
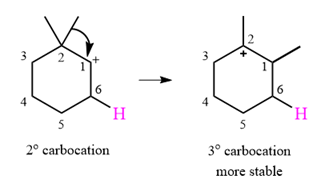
A
(e)
Interpretation:
Whether a carbocation rearrangement will take place in an
Concept introduction:
The first step in an
A carbocation may undergo rearrangement through a
Stability of carbocations increases as
Answer to Problem 8.52P
A carbocation rearrangement will take place in this case.
The curved arrow notation for this rearrangement is
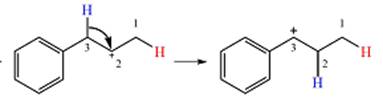
Explanation of Solution
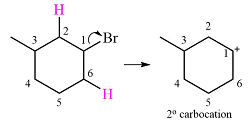
The given substrate initially forms the carbocation shown below in an
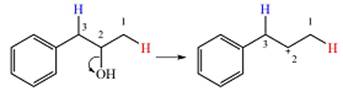
Two
The other, from C3 to C2 will result in another secondary carbocation. This would normally not lead to a more stable carbocation. However, in this case, the charge is now in a conjugated position with the
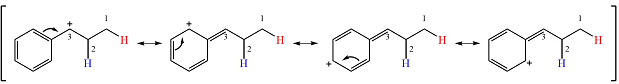
Therefore, a rearrangement will take place for this substrate.
The curved arrow notation for the rearrangement can be drawn as
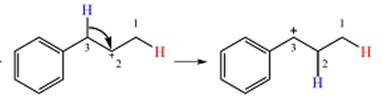
Resonance can increase the stability of a carbocation as the number of atoms on which the charge is delocalized increases.
(f)
Interpretation:
Whether a carbocation rearrangement will take place in an
Concept introduction:
The first step in an
A carbocation may undergo rearrangement through a
Stability of carbocations increases as
Answer to Problem 8.52P
A carbocation rearrangement will take place in this case.
The curved arrow notation for the rearrangement can be drawn as
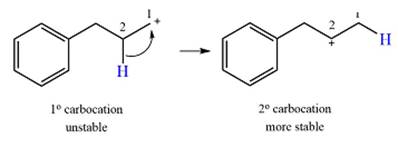
Explanation of Solution
The given substrate initially forms the carbocation, as shown below, in an
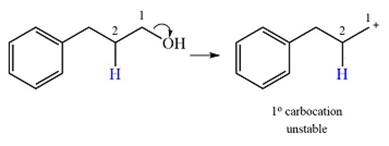
The carbocation initially formed is an unstable primary carbocation. Any rearrangement that converts it to a secondary or a tertiary carbocation will be favorable. There is only one possible
Therefore, a rearrangement of the carbocation will take place for this substrate.
The curved arrow notation for the rearrangement can be drawn as

A primary carbocation is unstable and will rearrange to a secondary carbocation where possible.
(g)
Interpretation:
Whether a carbocation rearrangement will take place in an
Concept introduction:
The first step in an
A carbocation may undergo rearrangement through a
Stability of carbocations increases as
Answer to Problem 8.52P
A carbocation rearrangement will take place in this case.
A curved arrow notation for the rearrangement can be drawn as
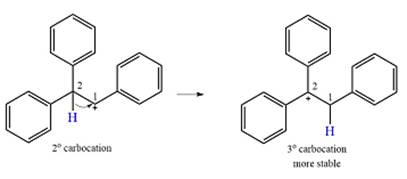
Explanation of Solution
The substrate and the carbocation that will initially be formed in an
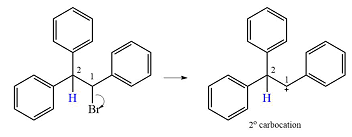
The carbocation initially formed is a relatively stable secondary carbocation. The presence of a benzene ring on the same carbon will lead to further stabilization by resonance. Resonance will delocalize the charge over a total of four carbon atoms.
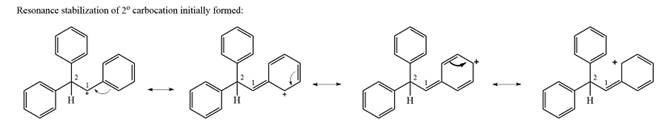
A
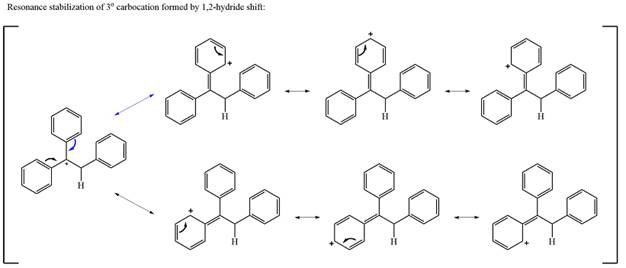
The much higher stability of this carbocation will mean a carbocation rearrangement will take place for this substrate.
The curved arrow notation for this rearrangement can be drawn as
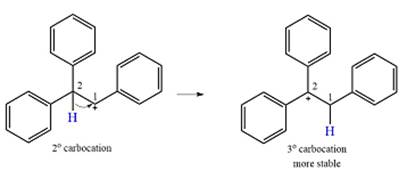
Formation of a more stable tertiary carbocation leads to the rearrangement.
(h)
Interpretation:
Whether a carbocation rearrangement will take place in an
Concept introduction:
The first step in an
A carbocation may undergo rearrangement through a
Stability of carbocations increases as
Answer to Problem 8.52P
No carbocation rearrangement will take place in this case.
Explanation of Solution
The given substrate and the carbocation that it forms initially in an
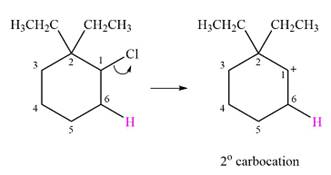
The carbocation formed is a relatively stable secondary carbocation. There is only one possible rearrangement, a
Therefore, a carbocation rearrangement will not take place in this case.
A carbocation rearrangement will not take place if it does not result in a more stable carbocation.
(i)
Interpretation:
Whether a carbocation rearrangement will take place in an
Concept introduction:
The first step in an
A carbocation may undergo rearrangement through a
Stability of carbocations increases as
Answer to Problem 8.52P
A carbocation rearrangement will take place in this case.
The curved arrow representation of this rearrangement can be drawn as
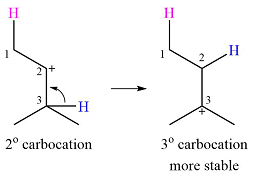
Explanation of Solution
The given substrate and the carbocation it will form in an
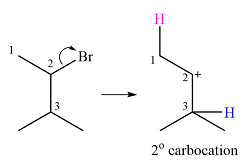
There are two possible
The second one, from C3 to C2, will result in a more stable tertiary carbocation.
Therefore, a carbocation rearrangement is possible in this case.
The curved arrow representation of this rearrangement can be drawn as

The carbocation rearrangement is possible because a more a stable carbocation is formed.
Want to see more full solutions like this?
Chapter 8 Solutions
Organic Chemistry: Principles And Mechanisms
- Draw a tetramer of this alternating copolymer.arrow_forwardH I T H HH H -H C. H- Identify and select all structures below that represent a constitutional isomer(s) of the compound shown above. H- H CIH H H H HHHH H H 0 ·H H– 冊 CH CHI HH C- H- H H- H H A. H H C H H- -H HH H B. H- -H D. H H H H • H -H E. -H H H HICH T HHH F. H-arrow_forwardPolylactic acid (shown below) is a biodegradable polymer used for food packaging. Identify the monomer(s) used in the production of this polymer using a condensation process.arrow_forward
- Draw the product of the reaction shown below. Ignore small byproducts that would evaporate pleasearrow_forwardPoly(ethylene adipate) is a biodegradable polyester (shown below). Identify the type of polymerization process used in the production of this polymer.arrow_forwardPolymers may be composed of thousands of monomers. draw two repeat units(dimer) of the polymer formed in this reaction. assume there are hydrogen atoms on the two ends of the dimer. ignore inorganic byproducts pleasearrow_forward
- Draw the product of the reaction shown below. Use a dash or wedge bond to indicate stereochemistry of substituents on asymmetric centers, Ignore inorganic byproductsarrow_forwardDraw the product of this reaction please. Ignore inorganic byproductsarrow_forwardOne of the pi molecular orbitals of 1,3-butadiene (CH2=CHCH=CH2) is shown below. Please identify the number of nodal planes perpendicular to the bonding axisarrow_forward
- Draw the monomers required to synthesize this condensation polymer please.arrow_forwardProvide the correct systematic name for the compound shown here. Please take into account the keyboard options belowarrow_forwardcurved arrows are used to illustrate the flow of electrons. using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s)arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

