
Concept explainers
(a)
Interpretation:
Complete
Concept introduction:
A bimolecular nucleophilic substitution (
Answer to Problem 8.43P
Complete
(i)
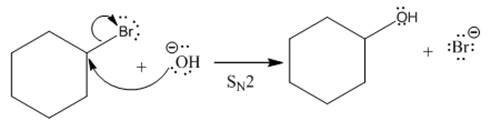
(ii)
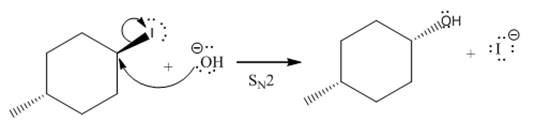
(iii)
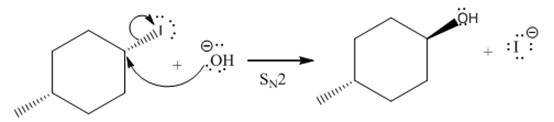
(iv)
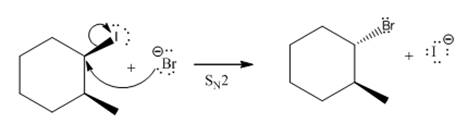
(v)
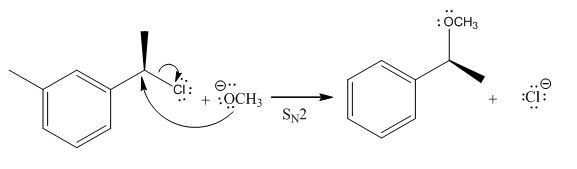
Explanation of Solution
(i)
The given reaction is
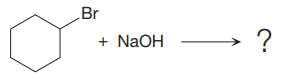
In the above reaction,
(ii)
The given reaction is
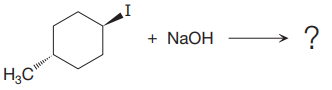
In the above reaction,
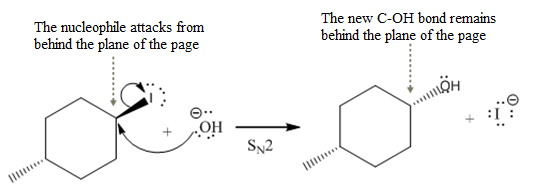
The stereochemistry of another group
(iii)
The given reaction
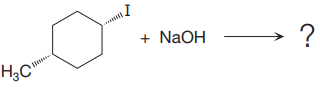
In the above reaction,
The stereochemistry of another group
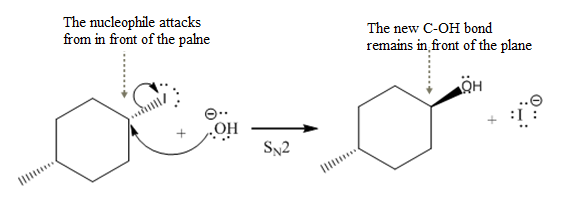
(iv)
The given reaction
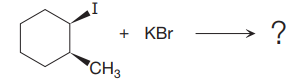
In the above reaction,
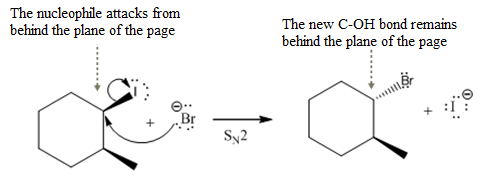
The stereochemistry of another group
(v)
The given reaction
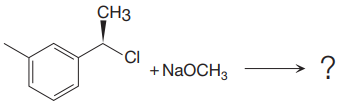
In the above reaction,
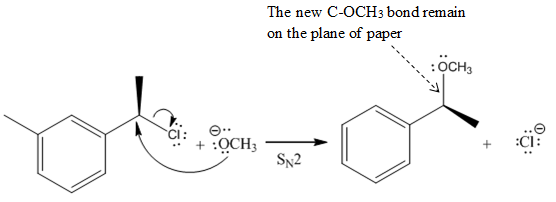
Another group
The Product of
(b)
Interpretation:
Complete
Concept introduction:
A unimolecular nucleophilic substitution (
If
Answer to Problem 8.43P
Complete
(i)
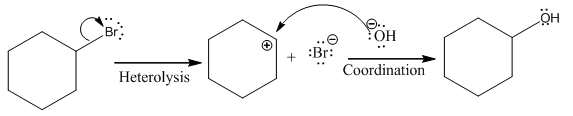
(ii)
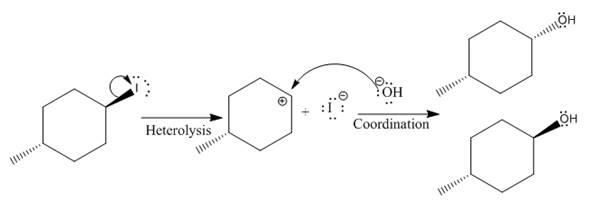
(iii)
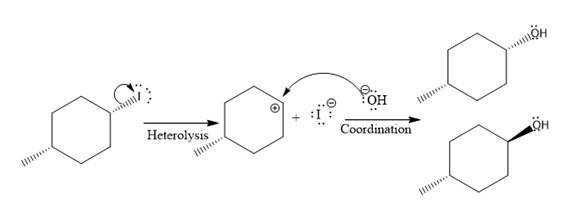
(iv)
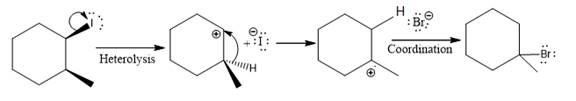
(v)
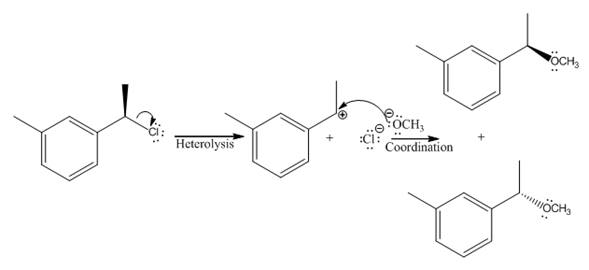
Explanation of Solution
(i)
The given reaction is
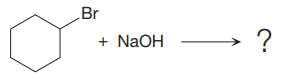

The leaving group in the above reaction is present on the plane of the planar, no stereochemistry is mentioned. Therefore, stereochemistry is not concerned at C, and the only isomer formed is the one shown in the above diagram.
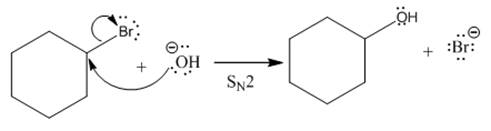
(ii)
The given reaction is
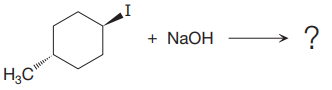
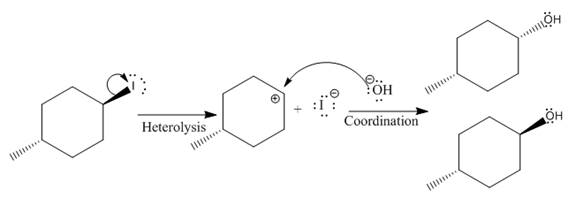
In the above reaction, leaving group (I) is present above the plane of the paper, thus the nucleophile attacks from both sides of the paper- behind the paper and in front of the paper resulting in the formation of two stereoisomers as shown above.
(iii)
The given reaction
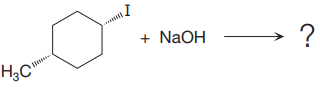

In the above reaction, leaving group (I) is present behind the plane of the paper, thus the nucleophile attacks from both sides of the paper- behind the paper and in front of the paper resulting in the formation of two stereoisomers as shown above.
(iv)
The given reaction
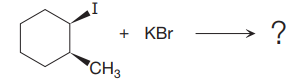
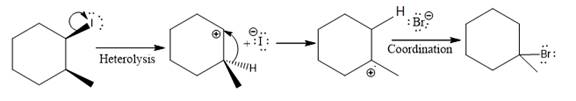
In the above reaction, the carbocation is stabilized by proton transfer. The new carbocation formed at the adjacent carbon from the leaving group is a more stable carbocation. The stereochemistry at the carbon (more stable carbocation) is of no concern. Therefore, the only isomer formed is as shown above.
(v)
The given reaction
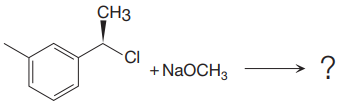
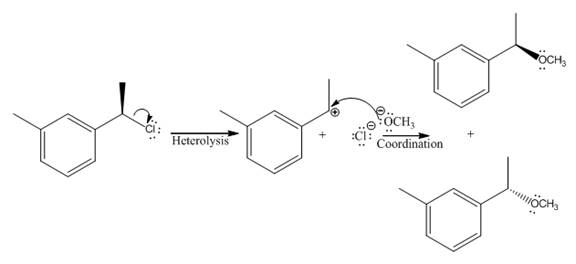
In the above reaction, chiral carbocation is formed once the leaving group leaves. Therefore, the nucleophile can attack from either side- from behind the paper and in front of the paper resulting in the formation of two stereoisomers as shown above.
Product of
Want to see more full solutions like this?
Chapter 8 Solutions
EBK ORGANIC CHEMISTRY: PRINCIPLES AND M
- Draw a tetramer of this alternating copolymer.arrow_forwardH I T H HH H -H C. H- Identify and select all structures below that represent a constitutional isomer(s) of the compound shown above. H- H CIH H H H HHHH H H 0 ·H H– 冊 CH CHI HH C- H- H H- H H A. H H C H H- -H HH H B. H- -H D. H H H H • H -H E. -H H H HICH T HHH F. H-arrow_forwardPolylactic acid (shown below) is a biodegradable polymer used for food packaging. Identify the monomer(s) used in the production of this polymer using a condensation process.arrow_forward
- Draw the product of the reaction shown below. Ignore small byproducts that would evaporate pleasearrow_forwardPoly(ethylene adipate) is a biodegradable polyester (shown below). Identify the type of polymerization process used in the production of this polymer.arrow_forwardPolymers may be composed of thousands of monomers. draw two repeat units(dimer) of the polymer formed in this reaction. assume there are hydrogen atoms on the two ends of the dimer. ignore inorganic byproducts pleasearrow_forward
- Draw the product of the reaction shown below. Use a dash or wedge bond to indicate stereochemistry of substituents on asymmetric centers, Ignore inorganic byproductsarrow_forwardDraw the product of this reaction please. Ignore inorganic byproductsarrow_forwardOne of the pi molecular orbitals of 1,3-butadiene (CH2=CHCH=CH2) is shown below. Please identify the number of nodal planes perpendicular to the bonding axisarrow_forward
- Draw the monomers required to synthesize this condensation polymer please.arrow_forwardProvide the correct systematic name for the compound shown here. Please take into account the keyboard options belowarrow_forwardcurved arrows are used to illustrate the flow of electrons. using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s)arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





