
CHEMISTRY-MOD.MASTERING (18W)
8th Edition
ISBN: 9780136780922
Author: Robinson
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 8, Problem 8.36CP
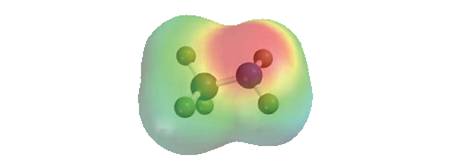
Methylarnine,
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Calculating the pH of a weak base titrated with a strong acid
An analytical chemist is titrating 184.2 mL of a 0.7800M solution of dimethylamine ((CH3)
NH with a 0.3000M solution of HClO4. The pK₁ of
dimethylamine is 3.27. Calculate the pH of the base solution after the chemist has added 424.1 mL of the HClO solution to it.
2
4
Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of HClO 4 solution added.
Round your answer to 2 decimal places.
pH = ☐
☑
?
000
18
Ar
1
B
Using the Nernst equation to calculate nonstandard cell voltage
A galvanic cell at a temperature of 25.0 °C is powered by the following redox reaction:
MnO2 (s)+4H* (aq)+2Cr²+ (aq) → Mn²+ (aq)+2H₂O (1)+2Cr³+ (aq)
+
2+
2+
3+
Suppose the cell is prepared with 7.44 M H* and 0.485 M Cr²+ in one half-cell and 7.92 M Mn² and 3.73 M Cr³+ in the other.
Calculate the cell voltage under these conditions. Round your answer to 3 significant digits.
☐
x10
μ
Х
5
?
000
日。
Calculating standard reaction free energy from standard reduction...
Using standard reduction potentials from the ALEKS Data tab, calculate the standard reaction free energy AG° for the following redox reaction.
Be sure your answer has the correct number of significant digits.
NO (g) +H₂O (1) + Cu²+ (aq) → HNO₂ (aq) +H* (aq)+Cu* (aq)
kJ
-
☐ x10
x10
olo
18
Ar
Chapter 8 Solutions
CHEMISTRY-MOD.MASTERING (18W)
Ch. 8 - Prob. 8.1PCh. 8 - What is the number and geometric arrangement of...Ch. 8 - PRACTICE 8.3 Acetic acid, CH3CO2H , is the main...Ch. 8 - APPLY 8.4 Benzene, C6H6 , is a cyclic molecule in...Ch. 8 - PRACTICE 8.5 Identify the orbitals that overlap to...Ch. 8 - APPLY 8.6 Describe the bonding in propane, C3H8 ,...Ch. 8 - PRACTICE 8.7 Describe the hybridization of the...Ch. 8 - Describe the hybridization of each carbon atom in...Ch. 8 - Which orbitals overlap to form the sigma and pi...Ch. 8 - APPLY 8.10 Describe the hybridization of the...
Ch. 8 - Prob. 8.11PCh. 8 - Conceptual APPLY 8.12 Match the following...Ch. 8 - Prob. 8.13PCh. 8 - Prob. 8.14ACh. 8 - Prob. 8.15PCh. 8 - Prob. 8.16ACh. 8 - Prob. 8.17ACh. 8 - Prob. 8.18ACh. 8 - The B2 molecule has a MO diagram similar to that...Ch. 8 - Prob. 8.20ACh. 8 - PRACTICE 8.23 Draw two resonance structures for...Ch. 8 - APPLY 8.24 Draw two resonance structures for the...Ch. 8 - Prob. 8.23PCh. 8 - Prob. 8.24PCh. 8 - Caffeine is the most widely used stimulant...Ch. 8 - Prob. 8.26PCh. 8 - What is the geometry around the central atom in...Ch. 8 - What is the geometry around the central atom in...Ch. 8 - Three of the following molecular models have a...Ch. 8 - Identify each of the following sets of hybrid...Ch. 8 - The VSEPR model is a simple predictive tool that...Ch. 8 - The following ball-and-stick molecular model is a...Ch. 8 - The following ball-and-stick molecular model is a...Ch. 8 - Prob. 8.34CPCh. 8 - The dipole moment of methanol is =1.70D . Use...Ch. 8 - Methylarnine, CH3NH2 , is responsible for the odor...Ch. 8 - Prob. 8.37CPCh. 8 - Prob. 8.38SPCh. 8 - What shape do you expect for molecules that meet...Ch. 8 - How many charge clouds are there around the...Ch. 8 - Prob. 8.41SPCh. 8 - What shape do you expect for each of the following...Ch. 8 - What shape do you expect for each of the following...Ch. 8 - What shape do you expect for each of the following...Ch. 8 - Prob. 8.45SPCh. 8 - Prob. 8.46SPCh. 8 - What shape do you expect for each of the following...Ch. 8 - What bond angles do you expect for each of the...Ch. 8 - What bond angles do you expect for each of the...Ch. 8 - Acrylonitrile is used as the starting material for...Ch. 8 - Predict values for all bond angles in dimethyl...Ch. 8 - Oceanographers study the mixing of water masses by...Ch. 8 - A potential replacement for the chlorofluorocarbon...Ch. 8 - Explain why cyclohexane, a substance that contains...Ch. 8 - Like cyclohexane (Problem 8.54), benzene also...Ch. 8 - Use VSEPR theory to answer the following...Ch. 8 - Draw an electron-dot structure for each of the...Ch. 8 - What is the difference in spatial distribution...Ch. 8 - The average CC bond dissociation energy (D) is 350...Ch. 8 - What hybridization do you expect for atoms that...Ch. 8 - What spatial arrangement of charge clouds...Ch. 8 - What hybridization would you expect for the...Ch. 8 - What hybridization would you expect for the...Ch. 8 - Oxaloacetic acid is an intermediate involved in...Ch. 8 - The atoms in the amino acid glycine are connected...Ch. 8 - Describe the hybridization of the carbon atom in...Ch. 8 - Describe the hybridization of each carbon atom in...Ch. 8 - Bupropion, marketed as Wellbutr in, is a heavily...Ch. 8 - Efavirenz, marketed as Sustiva, is a medication...Ch. 8 - What is the hybridization of the B and N atoms in...Ch. 8 - Prob. 8.71SPCh. 8 - Aspirin has the following connections among atoms....Ch. 8 - The cation [HCNXeF]+ is entirely linear. Draw an...Ch. 8 - Acrylonitrile (C3H3N) is a molecule that is...Ch. 8 - The odor of cinnamon oil is due to cinnamaldehyde,...Ch. 8 - The following molecular model is a representation...Ch. 8 - Prob. 8.77SPCh. 8 - Which of the following substances would you expect...Ch. 8 - Which of the following substances would you expect...Ch. 8 - Why is the dipole moment of SO2 1.63 D hut that of...Ch. 8 - Prob. 8.81SPCh. 8 - The class of ions PtX42 , where X is a halogen,...Ch. 8 - Prob. 8.83SPCh. 8 - Prob. 8.84SPCh. 8 - Prob. 8.85SPCh. 8 - Prob. 8.86SPCh. 8 - Prob. 8.87SPCh. 8 - What are the most important kinds of...Ch. 8 - Of the substances Xe, CH3Cl , and HF which has:...Ch. 8 - Methanol (CH3OH;bp=65C) boils nearly 230 °C higher...Ch. 8 - Prob. 8.91SPCh. 8 - Prob. 8.92SPCh. 8 - Prob. 8.93SPCh. 8 - A liquid sample contains methylamine (CH3NH2)...Ch. 8 - Prob. 8.95SPCh. 8 - What is the difference in spatial distribution...Ch. 8 - Prob. 8.97SPCh. 8 - Use the MO energy diagram in Figure 8.22b to...Ch. 8 - Use the MO energy diagram in Figure 8.22 a to...Ch. 8 - The C2 molecule can be represented by an MO...Ch. 8 - Prob. 8.101SPCh. 8 - Prob. 8.102SPCh. 8 - Prob. 8.103SPCh. 8 - Draw a molecular orbital energy diagram for Li2 ....Ch. 8 - Calcium carbide, CaC2 , reacts with water to...Ch. 8 - At high temperatures, sulfur vapor is...Ch. 8 - Carbon monoxide is produced by incomplete...Ch. 8 - Make a sketch showing the location and geometry of...Ch. 8 - Make a sketch showing the location and geometry of...Ch. 8 - Prob. 8.110MPCh. 8 - Prob. 8.111MPCh. 8 - Prob. 8.112MPCh. 8 - Prob. 8.113MPCh. 8 - Just as individual bonds in a molecule are often...Ch. 8 - Cyclooctatetraenedian ion, C8H82 , is an organic...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Calculating the pH of a weak base titrated with a strong acid b An analytical chemist is titrating 116.9 mL of a 0.7700M solution of aniline (C6H5NH2) with a 0.5300M solution of HNO3. The pK of aniline is 9.37. Calculate the pH of the base solution after the chemist has added 184.2 mL of the HNO 3 solution to it. Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of HNO3 solution added. Round your answer to 2 decimal places. pH = ☐ ☑ 5arrow_forwardQUESTION: Find the standard deviation for the 4 different groups 5.298 3.977 223.4 148.7 5.38 4.24 353.7 278.2 5.033 4.044 334.6 268.7 4.706 3.621 305.6 234.4 4.816 3.728 340.0 262.7 4.828 4.496 304.3 283.2 4.993 3.865 244.7 143.6 STDEV = STDEV = STDEV = STDEV =arrow_forwardQUESTION: Fill in the answers in the empty green boxes regarding 'Question 5: Calculating standard error of regression' *The images of the data showing 'coefficients for the standard curve' have been providedarrow_forward
- Using the Nernst equation to calculate nonstandard cell voltage Try Again Your answer is wrong. In addition to checking your math, check that you used the right data and DID NOT round any intermediate calculations. A galvanic cell at a temperature of 25.0 °C is powered by the following redox reaction: 2+ 2+ Sn²+ Ba(s) (aq) + Ba (s) Sn (s) + Ba²+ (aq) →>> Suppose the cell is prepared with 6.10 M Sn 2+ 2+ in one half-cell and 6.62 M Ba in the other. Calculate the cell voltage under these conditions. Round your answer to 3 significant digits. 1.71 V ☐ x10 ☑ 5 0/5 ? 00. 18 Ararrow_forwardQuestion: Find both the b (gradient) and a (y-intercept) value from the list of data below: (x1 -x̄) 370.5 (y1 - ȳ) 5.240 (x2 - x̄) 142.5 (y2 - ȳ) 2.004 (x3 - x̄) 28.5 (y3 - ȳ) 0.390 (x4 - x̄) -85.5 (y4 - ȳ) -1.231 (x5 - x̄) -199.5 (y5 - ȳ) -2.829 (x6 - x̄) -256.5 (y6 - ȳ) -3.575arrow_forwardCalculating standard reaction free energy from standard reduction... Using standard reduction potentials from the ALEKS Data tab, calculate the standard reaction free energy AG° for the following redox reaction. Be sure your answer has the correct number of significant digits. 3Cu+ (aq) + Cro²¯ (aq) +4H₂O (1) → 3Cu²+ (aq) +Cr(OH)3 (s)+5OH˜¯ (aq) 0 kJ ☐ x10 00. 18 Ararrow_forward
- Calculating the pH of a weak base titrated with a strong acid An analytical chemist is titrating 241.7 mL of a 0.4900M solution of methylamine (CH3NH2) with a 0.7800M solution of HNO3. The pK of methylamine is 3.36. Calculate the pH of the base solution after the chemist has added 17.7 mL of the HNO3 solution to it. Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of HNO3 solution added. Round your answer to 2 decimal places. pH = ☑ ? 18 Ararrow_forwardThe following is two groups (Regular tomato sauce & Salt Reduced Tomato Sauce) of data recorded by a team analysising salt content in tomato sauce using the MOHR titration method: Regular Tomato Sauce Salt Reduced Tomato Sauce 223.4 148.7 353.7 278.2 334.6 268.7 305.6 234.4 340.0 262.7 304.3 283.2 244.7 143.6 QUESTION: For both groups of data calculate the answers attached in the image.arrow_forwardThe following is a two groups (Regular tomato sauce & Salt Reduced Tomato Sauce) of data recorded by a team analysising salt content in tomato sauce using the MOHR titration method: Regular Tomato Sauce Salt Reduced Tomato Sauce 340.0mmol/L 262.7mmol/L QUESTION: For both groups (Regular & Salt Reduced tomato sauce) of data provide answers to the following calculations below: 1. Standard Deviation (Sx) 2. T Values (t0.05,4) 3. 95% Confidence Interval (mmol/L) 4. [Na+] (mg/100 mL) 5. 95% Confidence Interval (mg/100 mL)arrow_forward
- If we have leucine (2-amino-4-methylpentanoic acid), alanine (2-aminopropanoic acid) and phenylalanine (2-amino-3-phenylpropanoic acid), indicate the tripeptides that can be formed (use the abbreviated symbols Leu., Ala and Phe).arrow_forwardBriefly state why trifluoroacetic acid is more acidic than acetic acid.arrow_forwardExplain why acid chlorides are more reactive than amides in reactions with nucleophiles.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning


Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY