
EP ORGANIC CHEMISTRY-OWL V2 ACCESS
8th Edition
ISBN: 9781305582453
Author: Brown
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 8, Problem 8.27P
Interpretation Introduction
Interpretation:
Concept introduction:
Halogenation of
Radical chain reaction:
Initiation reaction: 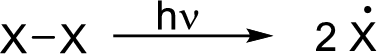
Chain propagation: 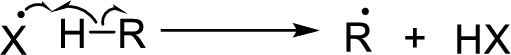
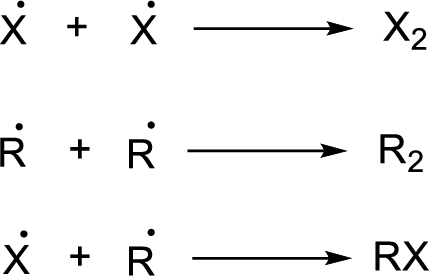
Chain termination:
It is a change in enthalpy of a homolysis reaction at absolute zero where a molecule is broken down into two free radicals.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Chapter 8 Solutions
EP ORGANIC CHEMISTRY-OWL V2 ACCESS
Ch. 8.2 - Prob. 8.1PCh. 8.4 - Name and draw structural formulas for all...Ch. 8.4 - Using the table of bond dissociation enthalpies in...Ch. 8.5 - Prob. 8.4PCh. 8.6 - Given the solution to Example 8.5, predict the...Ch. 8.7 - Prob. 8.6PCh. 8.7 - Linoleic acid is shown below. What makes this...Ch. 8.7 - Prob. BQCh. 8.7 - Prob. CQCh. 8.7 - The strength of the HO bond in vitamin E is weaker...
Ch. 8.7 - Prob. EQCh. 8.8 - Prob. 8.7PCh. 8 - Prob. 8.8PCh. 8 - Prob. 8.9PCh. 8 - Prob. 8.10PCh. 8 - Prob. 8.11PCh. 8 - Account for the fact that among the chlorinated...Ch. 8 - Name and draw structural formulas for all possible...Ch. 8 - Prob. 8.14PCh. 8 - There are three constitutional isomers with the...Ch. 8 - Following is a balanced equation for bromination...Ch. 8 - Prob. 8.17PCh. 8 - Prob. 8.18PCh. 8 - Prob. 8.19PCh. 8 - Cyclobutane reacts with bromine to give...Ch. 8 - Prob. 8.21PCh. 8 - Following is a balanced equation for the allylic...Ch. 8 - Prob. 8.23PCh. 8 - Prob. 8.24PCh. 8 - The major product formed when methylenecyclohexane...Ch. 8 - Prob. 8.26PCh. 8 - Prob. 8.27PCh. 8 - Prob. 8.28PCh. 8 - Write the products of the following sequences of...Ch. 8 - Using your reaction roadmap as a guide, show...Ch. 8 - Prob. 8.31PCh. 8 - Prob. 8.32PCh. 8 - Prob. 8.33PCh. 8 - Prob. 8.34P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Coenzymes and cofactors; Author: CH15 SWAYAM Prabha IIT Madras;https://www.youtube.com/watch?v=bubY2Nm7hVM;License: Standard YouTube License, CC-BY
Aromaticity and Huckel's Rule; Author: Professor Dave Explains;https://www.youtube.com/watch?v=7-BguH4_WBQ;License: Standard Youtube License