
(a)
Interpretation:
The two expressions for Doppler broadening and Doppler half-width needs to be shown equivalent to each other.
Concept introduction:
The equation for the half-width for Doppler broadening Δλ0 of an atomic line can be used to study line broadening in a low − pressure laser-induced plasma.
Explanation of Solution
The change in wavelength at the center of the emission line can be represented as follows:
Here,
Similarly, the Doppler half-width can be calculated as follows:
Here,
Also,
(b)
Interpretation:
The half-width for Doppler broadening needs to be determined for 4s to 4p transition for nickel atom.
Concept introduction:
Doppler bordering is happened due to the Doppler effect caused by a distribution of velocities of atomic molecules.
Answer to Problem 8.12QAP
The half-width = 7934 nm and
Explanation of Solution
Given information:
Calculation:
The Doppler half-width can be calculated as follows:
(c)
Interpretation:
The natural line width for the above transition needs to be determined, assuming that the lifetime of the excited state is
Concept introduction:
Natural line width is associated with the decay time (Natural life-time) and it is a minimum line width that does not contain effects such as collisional and Doppler broadening.
Answer to Problem 8.12QAP
Natural line width =
Explanation of Solution
Natural line width can be calculated as follows:
Putting the values,
(d)
Interpretation:
To show that the relativistic expression is consistent with the mentioned equation given for the low atomic speeds.
Concept introduction:
When compared with the
Explanation of Solution
When the atomic speed very low V is considerably small when compared to the c, that of the speed of light. Hence the above mentioned equation could be written as shown below. Hence, at low velocities, relativistic kinetic energy reduces to classical kinetic energy. No object with mass can achieve the speed of light because an infinite amount of energy input and an infinite amount of work is required to accelerate a mass to the speed of light.
(e)
Interpretation:
The speed of an iron atom the 4s to 4p transition at 385.9911 nm should be determined.
Concept introduction:
The rest wavelength of Nickel is 410 nm. The formula used is:
Answer to Problem 8.12QAP
Explanation of Solution
Given information:
Calculation:
(f)
Interpretation:
The fraction of a sample of iron atoms at 10,000 K that would have the velocity calculated in part e should be computed.
Concept introduction:
Natural line width is associated with the decay time. It is a minimum line width that does not contain effects such as collisional and Doppler broadening.
Answer to Problem 8.12QAP
Explanation of Solution
Given information:
Calculation:
(g)
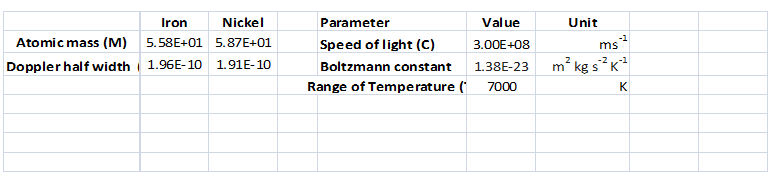
Interpretation:
A spreadsheet should be created to calculate the Doppler half-width
Concept introduction:
Doppler bordering is happened due to the Doppler effect caused by a distribution of velocities of atomic molecules.
Answer to Problem 8.12QAP
Refer the spreadsheet
Explanation of Solution
Given information:
Calculation:
(h)
Interpretation:
The four sources of pressure broadening should be listed by consulting the paper by Gornushkin et al. (note 10).
Explanation of Solution
The interaction of the surrounding particles with the radiating atom is the major source of pressure line broadening, which causes a phase shift and a frequency disturbance.
The most important cases of interaction are:
- linear Starkeffect, p = 2;
- resonance interaction between identical particles, p = 3;
- quadratic Stark effect, p = 4,
- van der Waals interaction, p = 6.
The superposition problems are avoided by two approximations:
- ‘nearest neighbor approximation’, in this the considered interaction is interaction with the closest perturber.
- The impact or collision concept, in which moving perturbers act sequentially in time.
Want to see more full solutions like this?
Chapter 8 Solutions
PRINCIPLES OF INSTRUMENTAL ANALYSIS
- presented by Morallen Lig Intermine the hand product for the given mution by adding atoms, bonds, nonhonding diarion panda скуль Step 3: Comp the draw the product Step 2: Agama workup Compithe 429 ملولةarrow_forwardReaction A 0,0arrow_forwardpresented by Morillon Leaning Predict the organic product for the min кусур HSC Adithane carved arnown to come than that to the condon slchroruis in acid in in aquishri with ноюarrow_forward
- 6.15PM Sun Mar 30 K Draw the major product of this reaction. Include any relevant stereochemistry. Ignore inorganic byproducts. Problem 1 of O H [PhзPCH2CH3]*C|¯ NaH Drawing > Q Atoms, Bonds and Draw or tap a nearrow_forward8:17 PM Sun Mar 30 Draw the major product of this reaction. Ignore inorganic byproducts. HSCH2CH2CH2SH, BF3 Probler Drawing Ato Bonds Clarrow_forwardpresented by Mr L How the coprion. (Il Done in no wraction, dew the starting redential) доarrow_forward
- 8:16 PM Sun Mar 30 K Draw the major product of this reaction. Ignore inorganic byproducts. Proble 1. CH3MgBr 2. H3O+ F Drawingarrow_forwardо но оarrow_forwardName the major organic product of the following action of 4-chloro-4-methyl-1-pentanol in neutral pollution 10+ Now the product. The product has a molecular formula f b. In a singly hain, the starting, material again converts into a secule with the molecular kormula CIO. but with comply Draw the major organic structure inhalationarrow_forward
- Macmillan Learning Alcohols can be oxidized by chromic acid derivatives. One such reagent is pyridinium chlorochromate, (C,H,NH*)(CICTO3), commonly known as PCC. Draw the proposed (neutral) intermediate and the organic product in the oxidation of 1-butanol by PCC when carried out in an anhydrous solvent such as CH₂C₁₂. PCC Intermediate OH CH2Cl2 Draw the intermediate. Select Draw Templates More с H Cr о Product Draw the product. Erase Select Draw Templates More H о Erasearrow_forwardIf I have 1-bromopropene, to obtain compound A, I have to add NaOH and another compound. Indicate which compound that would be. A C6H5 CH3arrow_forwardProvide the reagents for the following reactions.arrow_forward
 Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning


