
Owlv2 With Ebook, 1 Term (6 Months) Printed Access Card For Kotz/treichel/townsend/treichel's Chemistry & Chemical Reactivity, 10th
10th Edition
ISBN: 9781337791182
Author: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 8, Problem 79GQ
Hydroxyproline is a less-common amino acid.
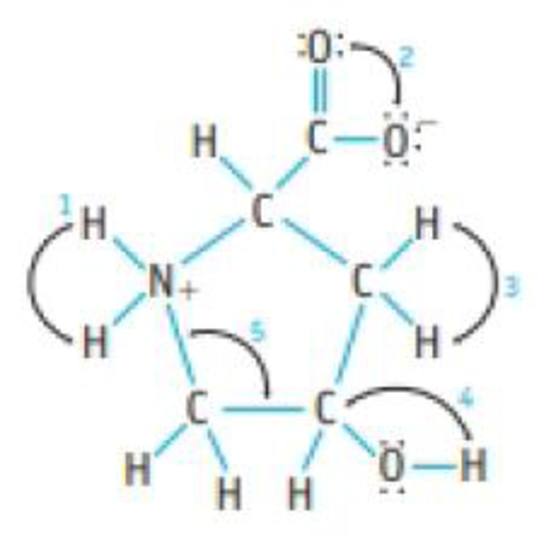
- (a) Give approximate values for the indicated bond angles.
- (b) Which are the most polar bonds in the molecule?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
At an electrified interface according to the Gouy-Chapman model, what types of interactions do NOT occur between the ions and the solvent according to this theory?
Please predict the products for each of the
following reactions.
Clearly show the regiochemistry (Markovnikov
vs anti-Markovnikov) and stereochemistry
(syn- vs anti- or both).
If a mixture of enantiomers is formed, please
draw all the enantiomers.
Hint: In this case you must choose the best
answer to demonstrate the stereochemistry of
H2 addition.
1.03
2. (CH3)2S
BIZ
CH₂OH
2. DMS
KMnO4, NaOH
ΖΗ
Pd or Pt (catalyst)
HBr
20 1
HBr
ROOR (peroxide)
HO
H-SO
HC
12 11 10
BH, THE
2. H2O2, NaOH
Brz
cold
HI
19
18
17
16
MCPBA
15
14
13
A
Br
H₂O
BH3⚫THF
Brz
EtOH
Pd or Ni (catalyst)
D₂ (deuterium)
1. Os04
2. H2O2
CH3CO3H
(peroxyacid)
1. MCPBA
2. H₂O*
H
B
+
H
H
H
"H
C
H
H
D
Explain how Beer’s Law can be used to determine the concentration in a selected food sample. Provide examples.
Chapter 8 Solutions
Owlv2 With Ebook, 1 Term (6 Months) Printed Access Card For Kotz/treichel/townsend/treichel's Chemistry & Chemical Reactivity, 10th
Ch. 8.2 - Draw Lewis electron dot structures for CH3Cl...Ch. 8.2 - Prob. 8.2CYUCh. 8.2 - Prob. 8.3CYUCh. 8.2 - Prob. 8.4CYUCh. 8.3 - Prob. 8.5CYUCh. 8.4 - Draw resonance structures for the bicarbonate ion,...Ch. 8.5 - Sketch the Lewis structures for CIF2+ and CIF2....Ch. 8.6 - What is the shape of the dichloromethane (CH2C12)...Ch. 8.6 - Give the electron-pair geometry and molecular...Ch. 8.6 - Draw the Lewis structure for lCl2, and then decide...
Ch. 8.7 - For each of the following pairs of bonds, decide...Ch. 8.7 - Draw the resonance structures for SCN. What are...Ch. 8.8 - For each of the following molecules, decide...Ch. 8.8 - The electrostatic potential surface for SOCl2 is...Ch. 8.9 - Using the bond dissociation enthalpies in Table...Ch. 8.10 - Prob. 1.1ACPCh. 8.10 - Do any of the atoms in an ibuprofen molecule have...Ch. 8.10 - What is the most polar bond in the molecule?
Ch. 8.10 - Prob. 1.4ACPCh. 8.10 - Prob. 1.5ACPCh. 8.10 - Prob. 1.6ACPCh. 8.10 - Are there any 120° bond angles in ibuprofen? Any...Ch. 8.10 - Prob. 1.8ACPCh. 8.10 - Prob. 2.2ACPCh. 8.10 - Calculate the difference in electronegativity...Ch. 8.10 - Predict the bond dissociation enthalpy for a...Ch. 8.10 - Prob. 3.3ACPCh. 8 - Give the periodic group number and number of...Ch. 8 - Give the periodic group number and number of...Ch. 8 - For elements in Groups 4A-7A of the periodic...Ch. 8 - Prob. 4PSCh. 8 - Draw a Lewis structure for each of the following...Ch. 8 - Draw a Lewis structure for each of the following...Ch. 8 - Draw a Lewis structure for each of the following...Ch. 8 - Draw a Lewis structure for each of the following...Ch. 8 - Show all possible resonance structures for each of...Ch. 8 - Show all possible resonance structures for each of...Ch. 8 - Prob. 11PSCh. 8 - Draw a Lewis structure for each of the following...Ch. 8 - Determine the formal charge on each atom in the...Ch. 8 - Determine the formal charge on each atom in the...Ch. 8 - Determine the formal charge on each atom in the...Ch. 8 - Determine the formal charge on each atom in the...Ch. 8 - Draw a Lewis structure for each of the following...Ch. 8 - Prob. 18PSCh. 8 - Prob. 19PSCh. 8 - The following molecules or ions all have three...Ch. 8 - Draw a Lewis structure for each of the following...Ch. 8 - Draw a Lewis structure for each of the following...Ch. 8 - Give approximate values for the indicated bond...Ch. 8 - Give approximate values for the indicated bond...Ch. 8 - Phenylalanine is one of the natural amino acids...Ch. 8 - Acetylacetone has the structure shown here....Ch. 8 - For each pair of bonds, indicate the more polar...Ch. 8 - For each of the bonds listed below, tell which...Ch. 8 - Urea, (NH2)2CO, is used in plastics and...Ch. 8 - Considering both formal charges and bond...Ch. 8 - Considering both formal charge and bond...Ch. 8 - Three resonance structures are possible for...Ch. 8 - Three resonance structures are possible for the...Ch. 8 - Compare the electron dot structures of the...Ch. 8 - Compare the electron dot structures of the...Ch. 8 - The chemistry of the nitrite ion and HNO2: (a) Two...Ch. 8 - Draw the resonance structures for the formate ion,...Ch. 8 - Prob. 39PSCh. 8 - Consider the following molecules: (a) CH4 (b)...Ch. 8 - Which of the following molecules is(are) polar?...Ch. 8 - Prob. 42PSCh. 8 - Give the bond order for each bond in the following...Ch. 8 - Prob. 44PSCh. 8 - In each pair of bonds, predict which is shorter....Ch. 8 - In each pair of bonds, predict which is shorter....Ch. 8 - Prob. 47PSCh. 8 - Compare the carbon-oxygen bond lengths in the...Ch. 8 - Consider the carbon-oxygen bond in formaldehyde...Ch. 8 - Compare the nitrogen-nitrogen bond in hydrazine,...Ch. 8 - Ethanol can be made by the reaction of ethylene...Ch. 8 - Methanol can be made by partial oxidation of...Ch. 8 - Hydrogenation reactions, which involve the...Ch. 8 - Phosgene, Cl2CO, is a highly toxic gas that was...Ch. 8 - The compound oxygen difluoride is quite reactive,...Ch. 8 - Oxygen atoms can combine with ozone to form...Ch. 8 - Prob. 57GQCh. 8 - Prob. 58GQCh. 8 - Which of the following compounds or ions do not...Ch. 8 - Prob. 60GQCh. 8 - Draw resonance structures for the formate ion,...Ch. 8 - Prob. 62GQCh. 8 - Prob. 63GQCh. 8 - What is the principle of electroneutrality? Use...Ch. 8 - Prob. 65GQCh. 8 - Draw resonance structures for the SO2 molecule,...Ch. 8 - What are the orders of the NO bonds in NO2 and...Ch. 8 - Which has the greater ONO bond angle, NO2 or NO2+?...Ch. 8 - Compare the FClF angles in CIF2+ and ClF2. Using...Ch. 8 - Draw an electron dot structure for the cyanide...Ch. 8 - Draw the electron dot structure for the sulfite...Ch. 8 - Dinitrogen monoxide, N2O, can decompose to...Ch. 8 - The equation for the combustion of gaseous...Ch. 8 - The cyanate ion, OCN, has the least...Ch. 8 - Vanillin is the flavoring agent in vanilla extract...Ch. 8 - Explain why (a) XeF2 has a linear molecular...Ch. 8 - The formula for nitryl chloride is ClNO2 (in which...Ch. 8 - Hydroxyproline is a less-common amino acid. (a)...Ch. 8 - Amides are an important class of organic...Ch. 8 - Prob. 81GQCh. 8 - The molecule shown here. 2-furylmelhanethiol, is...Ch. 8 - Dihydroxyacetone is a component of quick-tanning...Ch. 8 - It is possible to draw three resonance structures...Ch. 8 - Acrolein is used to make plastics. Suppose this...Ch. 8 - Molecules in space: (a) In addition to molecules...Ch. 8 - 1,2-Dichloroethylene can be synthesized by adding...Ch. 8 - The molecule pictured below is epinephrine, a...Ch. 8 - You are doing an experiment in the laboratory and...Ch. 8 - Prob. 90ILCh. 8 - A paper published in the research Journal Science...Ch. 8 - Uracil is one of the bases in RNA, a close...Ch. 8 - Guanine is present in both DNA and RNA. (a) What...Ch. 8 - Prob. 94ILCh. 8 - Prob. 95SCQCh. 8 - Prob. 96SCQCh. 8 - Bromine-containing species play a role in...Ch. 8 - Acrylamide, H2C=CHCONH2, is a known neurotoxin and...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Explain the importance of having a sampling plan with respect to food analysis. Explain the importance of having a sampling plan with respect to food analysis. Provide examples.arrow_forwardPlease predict the products for each of the following reactions. Clearly show the regiochemistry (Markovnikov vs anti-Markovnikov) and stereochemistry (syn- vs anti- or both). If a mixture of enantiomers is formed, please draw all the enantiomers. cold KMnO4, NaOH 2. DMS 1. 03 CH3OH Br2 1. 03 2. (CH3)2S H₂ Pd or Pt (catalyst) HBr 18 19 20 1 HBr ROOR (peroxide) H₂O H₂SO4 HCI HI 17 16 6 15 MCPBA 1. BH3 THF 2. H₂O2, NaOH 1. OsO4 2. H₂O₂ 110 CH3CO₂H (peroxyacid) 1. MCPBA 2. H₂O* Br2 H₂O BH3 THF B12 EtOH Pd or Ni (catalyst) D₂ (deuterium) Bra A B C D H OH H OH OH H OH α α α OH H OH OH фон d H "Harrow_forwardBriefly indicate the models that describe the structure of the interface: Helmholtz-Perrin, Gouy-Chapman, Stern and Grahame models.arrow_forward
- Using Benzene as starting materid show how each of the Following molecules Contel Ve syntheswed CHI 9. b -50311 с CHY 503H Ночто d. อ •NOV e 11-0-650 NO2arrow_forwardThe molecule PYRIDINE, 6th electrons and is therefore aromatre and is Assigned the Following structure contering Since aromatk moleculoy undergo electrophilic anomatic substitution, Pyridine shodd undergo The Following reaction + HNO3 12504 a. write all of the possible Mononitration Products that could Result From this reaction 18. Bared upon the reaction mechanison determime which of these producty would be the major Product of the hegetionarrow_forwarda. Explain Why electron withdrawing groups tend to be meta-Directors. Your answer Should lyclude all apropriate. Resonance contributing Structures fo. Explain why -ll is an outho -tura drccton even though chlorine has a very High Electronegativityarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY